Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D00DEF
|
||||
| Former ID |
DAP000571
|
||||
| Drug Name |
Lisdexamfetamine
|
||||
| Synonyms |
NRP104; Lisdexamfetamine (INN); Vyvanse (TN)
|
||||
| Drug Type |
Small molecular drug
|
||||
| Company |
New River Pharmaceuticals
|
||||
| Structure |
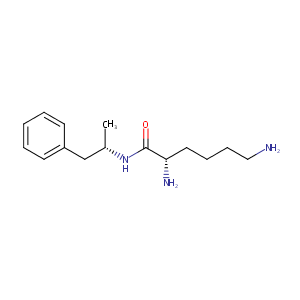
|
Download2D MOL |
|||
| Formula |
C15H25N3O
|
||||
| InChI |
InChI=1S/C15H25N3O/c1-12(11-13-7-3-2-4-8-13)18-15(19)14(17)9-5-6-10-16/h2-4,7-8,12,14H,5-6,9-11,16-17H2,1H3,(H,18,19)/t12-,14-/m0/s1
|
||||
| InChIKey |
VOBHXZCDAVEXEY-JSGCOSHPSA-N
|
||||
| CAS Number |
CAS 608137-32-2
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| SuperDrug ATC ID |
N06BA12
|
||||
| Target and Pathway | |||||
| Target(s) | Trace amine-associated receptor-1 | Target Info | Modulator | [529282], [531678] | |
| KEGG Pathway | Neuroactive ligand-receptor interaction | ||||
| Reactome | G alpha (s) signalling events | ||||
| References | |||||
| Ref 538579 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 021977. | ||||
| Ref 542228 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7213). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

