Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D05HFY
|
||||
| Former ID |
DAP000772
|
||||
| Drug Name |
Acenocoumarol
|
||||
| Synonyms |
Acenocoumarin; Acenocoumarolum; Acenocumarol; Acenocumarolo; Acenocumarolum; Acenokumarin; Ascumar; Minisintrom; Neositron;Nicoumalone; Nicumalon; Nitrovarfarian; Nitrowarfarin; Sincoumar; Sinkumar; Sinthrom; Sinthrome; Sintrom; Sintroma; Syncoumar; Syncumar; Synthrom; Syntrom; Zotil; Acenocoumarol Alliance Brand; Acenocoumarol Novartis Brand; Acenocoumarol [INN]; Acenocumarolo [DCIT]; Acenokumarin [Czech]; Alliance Brand of Acenocoumarol; Ciba Geigy Brand of Acenocoumarol; Mini Sintrom; Novartis Brand of Acenocoumarol; G 23350; Acenocoumarol (INN); Acenocoumarol Ciba-Geigy Brand; Acenocoumarolum [INN-Latin]; Ciba-Geigy Brand of Acenocoumarol; G-23350; Mini-sintrom; Sinthrome (TN); Sintrom (TN); AB-014/25000129; G-23,350; Mini-sintrom (TN); Nitrophenylacetylethyl-4-hydroxycoumarine; 2-hydroxy-3-[1-(4-nitrophenyl)-3-oxobutyl]-4h-chromen-4-one; 2-hydroxy-3-[1-(4-nitrophenyl)-3-oxobutyl]chromen-4-one; 3-(alpha-(4'-Nitrophenyl)-beta-acetylethyl)-4-hydroxycoumarin; 3-(alpha-(p-Nitrophenol)-beta-acetylethyl)-4-hydroxycoumarin; 3-(alpha-Acetonyl-4-nitrobenzyl)-4-hydroxycoumarin; 3-(alpha-Acetonyl-p-nitrobenzyl)-4-hydroxycoumarin; 3-(alpha-Acetonyl-para-nitrobenzyl)-4-hydroxy-coumarin; 3-(alpha-p-Nitrophenyl-beta-acetylethyl)-4-hydroxycoumarin; 4-Hydroxy-3-(1-(4-nitrophenyl)-3-oxobutyl)-2H-1-benzopyran-2-one;4-Hydroxy-3-[1-(4-nitrophenyl)-3-oxobutyl]-2H-chromen-2-one
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Thrombosis [ICD9: 437.6, 453, 671.5, 671.9; ICD10:I80-I82] | Approved | [550766] | ||
| Therapeutic Class |
Anticoagulants
|
||||
| Structure |
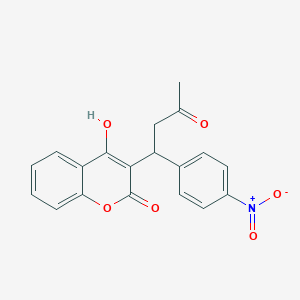
|
Download2D MOL |
|||
| Formula |
C19H15NO6
|
||||
| CAS Number |
CAS 152-72-7
|
||||
| PubChem Compound ID | |||||
| SuperDrug ATC ID |
B01AA07
|
||||
| SuperDrug CAS ID |
cas=000152727
|
||||
| Drug Resistance Mutation (DRM) | |||||
| DRM | DRM Info | ||||
| Target and Pathway | |||||
| Target(s) | Vitamin K epoxide reductase complex subunit 1 | Target Info | Inhibitor | [536520], [537085], [537545] | |
| KEGG Pathway | Ubiquinone and other terpenoid-quinone biosynthesis | ||||
| PathWhiz Pathway | Vitamin K Metabolism | ||||
| Coagulation | |||||
| References | |||||
| Ref 536520 | Oral anticoagulation and pharmacogenetics: importance in the clinical setting. Rev Med Suisse. 2007 Sep 12;3(124):2030, 2033-4, 2036. | ||||
| Ref 537085 | Genotypes associated with reduced activity of VKORC1 and CYP2C9 and their modification of acenocoumarol anticoagulation during the initial treatment period. Clin Pharmacol Ther. 2009 Apr;85(4):379-86. Epub 2009 Feb 18. | ||||
| Ref 537545 | Evaluation of a reverse-hybridization StripAssay for the detection of genetic polymorphisms leading to acenocoumarol sensitivity. Mol Biol Rep. 2009 Jun 28. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

