Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01AJY
|
||||
| Former ID |
DAP000257
|
||||
| Drug Name |
Baclofen
|
||||
| Synonyms |
ApoBaclofen; Atrofen; Baclofene; BaclofeneIrex; Baclofeno; Baclofenum; Baclon; Baclophen; Baclospas; Clofen; Gabalon; GenBaclofen; Genpharm; Kemstro; Lebic; Lioresal; NuBaclo; PMSBaclofen; ASTA Medica Brand of Baclofen; Alphapharm Brand of Baclofen; Apo Baclofen; Apotex Brand of Baclofen; Ashbourne Brand of Baclofen; Athena Brand of Baclofen; Baclofen AWD; Baclofen Alphapharm Brand; Baclofen Apotex Brand; Baclofen Ashbourne Brand; Baclofen Athena Brand; Baclofen Irex Brand; Baclofen Isis Brand; Baclofen Medtronic Brand; Baclofen Novartis Brand; Baclofen Pharmascience Brand; Baclofene Irex; Chlorophenyl GABA; Ciba Geigy Brand of Baclofen; Gen Baclofen; Irex Brand of Baclofen; Isis Brand of Baclofen; Lioresal Intrathecal; Medtronic Brand of Baclofen; Novartis Brand of Baclofen; Nu Baclo; Nu Pharm Brand of Baclofen; PMS Baclofen; Pharmascience Brand of Baclofen; B 5399; Ba 34647; Ba34647; C 34647Ba; AWD, Baclofen; Apo-Baclofen; Ba-34647; Ba34,647; Baclofen Ciba-Geigy Brand; Baclofen Nu-Pharm Brand; Baclofene [INN-French]; Baclofene-Irex; Baclofeno [INN-Spanish]; Baclofenum [INN-Latin]; CIBA34,647BA; Ciba-Geigy Brand of Baclofen; DL-Baclofen; GABA, Chlorophenyl; Gen-Baclofen; Kemstro (TN); Lioresal (TN); Nu-Baclo; Nu-Baclofen; Nu-Pharm Brand of Baclofen; PCP-GABA; Pms-Baclofen; Ba-34,647; Baclofen (R,S); Ciba 34,647-Ba; Baclofen (JP15/USP/INN); Baclofen [USAN:INN:BAN:JAN]; Beta-(4-Chlorophenyl)gaba; CIBA-34,647-BA; Beta-(Aminomethyl)-4-chlorobenzenepropanoic acid; Beta-(Aminomethyl)-p-chlorohydrocinnamic acid; Beta-(p-Chlorophenyl)-gamma-aminobutyric acid; DL-4-Amino-3-p-chlorophenylbutanoic acid; Gamma-Amino-beta-(p-chlorophenyl)butyric acid; Benzenepropanoic acid, beta-(aminomethyl)-4-chloro-(9CI); (+-)-Baclofen; (+/-)-BACLOFEN; (+/-)-beta-(Aminoethyl)-4-chlorobenzenepropanoic acid; (+/-)-beta-(Aminomethyl)-4-chlorobenzenepropanoic acid; (inverted question mark)-Baclofen; 4-Amino-3-(4-chlorophenyl)butanoic acid; 4-Amino-3-(4-chlorophenyl)butyric acid
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Muscle Relaxants
|
||||
| Company |
Norvatis Phamaceuticals Corporation
|
||||
| Structure |
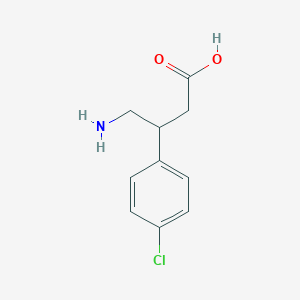
|
Download2D MOL |
|||
| Formula |
C10H12ClNO2
|
||||
| InChI |
InChI=1S/C10H12ClNO2/c11-9-3-1-7(2-4-9)8(6-12)5-10(13)14/h1-4,8H,5-6,12H2,(H,13,14)/t8-/m0/s1
|
||||
| InChIKey |
KPYSYYIEGFHWSV-QMMMGPOBSA-N
|
||||
| CAS Number |
CAS 1134-47-0
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
3153571, 8178796, 11113710, 15121255, 15171983, 34709846, 50066102, 50705527, 90452213, 93625490, 103238751, 103941336, 104345301, 121277848, 123055284, 124490531, 124580859, 126581462, 126616647, 126685003, 126738367, 128418031, 134351756, 135249532, 135649977, 139309014, 143493644, 152032454, 152165673, 152238531, 152258754, 160647599, 160840514, 162009782, 162255234, 163099671, 163405182, 166222624, 170475242, 170485722, 172914818, 175266631, 175270364, 178100431, 186007019, 198993730, 204431203, 208265328, 223518884, 223679055
|
||||
| ChEBI ID |
ChEBI:2972
|
||||
| SuperDrug ATC ID |
M03BX01
|
||||
| SuperDrug CAS ID |
cas=001134470
|
||||
| Target and Pathway | |||||
| Target(s) | GABA(B) receptor | Target Info | Modulator | [556264] | |
| References | |||||
| Ref 536096 | Development of medications for alcohol use disorders: recent advances and ongoing challenges. Expert Opin Emerg Drugs. 2005 May;10(2):323-43. | ||||
| Ref 538665 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1084). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

