Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0U4UQ
|
||||
| Former ID |
DAP000750
|
||||
| Drug Name |
Hydrochlorothiazide
|
||||
| Synonyms |
Acesistem; Acuilix; Acuretic; Aldazida; Aldoril; Apresazide; Aquarills; Aquarius; Bremil; Briazide; Caplaril; Carozide; Catiazida; Chlorizide; Chlorosulthiadil; Chlorzide; Chlothia; Cidrex; Clorana; Condiuren; Diaqua; Dichlorosal; Dichlorotride; Dichlothiazide; Dichlotiazid; Dichlotride; Diclotride; Dicyclotride; Didral; Dihydran; Dihydrochlorothiazid; Dihydrochlorothiazide; Dihydrochlorothiazidum; Dihydrochlorurit; Dihydrochlorurite; Dihydroxychlorothiazidum; Direma; Disalunil; Disothiazid; Diurogen; Dixidrasi; Drenol; Esidrex; Esidrix; Esoidrina; Fluvin; HCT; HCTZ; HCZ; Hidril; Hidrochlortiazid; Hidroclorotiazida; Hidroronol; Hidrosaluretil; Hidrotiazida; Hyclosid; Hydril; HydroDIURIL; Hydrochlorat; Hydrochlorot; Hydrochlorothiazid; Hydrochlorothiazidum; Hydrochlorthiazide; Hydrochlorthiazidum; Hydrocot; Hydrodiuretic; Hydropres; Hydrosaluric; Hydrothide; Hydrozide; Hypothiazid; Hypothiazide; Hytrid; Idroclorotiazide; Idrotiazide; Indroclor; Ivaugan; Manuril; Maschitt; Medozide; Megadiuril; Microzide; Mictrin; Mikorten; Modurcen; Moduretic; Natrinax; Nefrix; Nefrol; Neoflumen; Newtolide; Novodiurex; Oretic; Pantemon; Panurin; Roxane; Saldiuril; Sectrazide; Selozide; Servithiazid; Spironazide; Tandiur; Thiaretic; Thiuretic; Thlaretic; Timolide; Unazid; Urodiazin; Urozide; Vaseretic; Vetidrex; Aquazide H; Chlorsulfonamidodihydrobenzothiadiazine dioxide; Component of Aldactazide; Component of Aldoril; Component of Butizide Prestabs; Component of Caplaril; Component of Cyclex; Component of Dyazide; Component of Esimil; Concor Plus; Diclot ride; Hydro Par; Hydrochlorothiazide Intensol; Idroclorotiazide [DCIT]; Lotensin HCT; Panurin dichloride; Raunova Plus; Diu 25 Vigt; H 4759; MaybridgeCompound 10; Mazide 25 mg; Su 5879; Aldactazide 25/25; Aldectazide 50/50; Apo-Hydro; Aquazide H (TN); Aquazide-H; Dichlotride (TN); Diu-melusin; Esidrex (TN); Esidrix (TN); HCT-Isis; Hidro-Niagrin; Hidroclorotiazida [INN-Spanish]; Hydrex-semi; Hydro-Aquil; Hydro-D; Hydro-Diuril; Hydro-Saluric; Hydro-T; Hydro-chlor; HydroSaluric (TN); Hydrochlorothiazidum [INN-Latin]; Hydrodiuril (TN); Hydrozide Injection, Veterinary; Inderide 80/25; Jen-Diril; Microzide (TN); Neo-Flumen; Neo-Minzil; Neo-codema; Oretic (TN); Ro-hydrazide; AF-614/30832002; Apo-Hydro (TN); Hydrochlorothiazide [INN:BAN:JAN]; Adiazine-1,1-dioxide; Hydrochlorothiazide (JP15/USP/INN); Dro-2H-1,2,4-benzothiadiazine 1,1-dioxide; 3,4-Dihydro-6-chloro-7-sulfamyl-1,2, 4-benzothi; 3,4-Dihydrochlorothiazide; 6-Chloro-7-sulfamoyl-3, 4-dihy
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Diuretics
|
||||
| Structure |
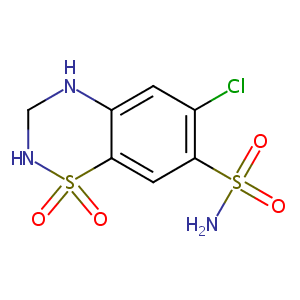
|
Download2D MOL |
|||
| Formula |
C7H8ClN3O4S2
|
||||
| InChI |
InChI=1S/C7H8ClN3O4S2/c8-4-1-5-7(2-6(4)16(9,12)13)17(14,15)11-3-10-5/h1-2,10-11H,3H2,(H2,9,12,13)
|
||||
| InChIKey |
JZUFKLXOESDKRF-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 58-93-5
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9253, 103995, 220766, 855646, 1434462, 3159668, 5137564, 7847406, 7979544, 8149374, 8152296, 10321359, 10528484, 10589262, 11111269, 11111270, 11335464, 11360703, 11363771, 11366333, 11368895, 11371452, 11373490, 11377057, 11382607, 11408708, 11461675, 11466037, 11467157, 11484995, 11485674, 11488856, 11490272, 11491821, 11494691, 14873640, 16977795, 17389854, 17405176, 24277806, 24895542, 26611772, 26680075, 26746944, 26746945, 26751473, 29222764, 46504440, 46513493, 47216682
|
||||
| SuperDrug ATC ID |
C03AA03
|
||||
| SuperDrug CAS ID |
cas=000058935
|
||||
| Target and Pathway | |||||
| Target(s) | Angiotensin-converting enzyme | Target Info | Modulator | [556264] | |
| PathWhiz Pathway | Angiotensin Metabolism | ||||
| References | |||||
| Ref 468069 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4836). | ||||
| Ref 538179 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 040412. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

