Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D03DAP
|
||||
| Former ID |
DAP000518
|
||||
| Drug Name |
Flecainide
|
||||
| Synonyms |
Flecaine; Flecainida; Flecainidum; Tambocor; Almarytm (TN); Apocard (TN); Ecrinal (TN); Flecainida [INN-Spanish]; Flecainide (INN); Flecainide [INN:BAN]; Flecainidum [INN-Latin]; Tambocor (TN); N-(2-Piperidinylmethyl)-2,5-bis(2,2,2-trifluoroethoxy)benzamide; N-(piperidin-2-ylmethyl)-2,5-bis(2,2,2-trifluoroethoxy)benzamide; N-(piperidin-2-ylmethyl)-2,5-bis[(2,2,2-trifluoroethyl)oxy]benzamide; (+-)-Flecainide
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antiarrhythmic Agents
|
||||
| Company |
3M pharmaceuticals
|
||||
| Structure |
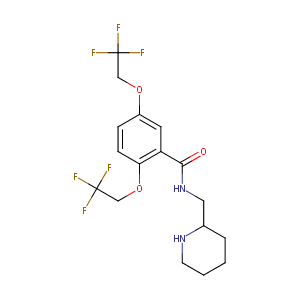
|
Download2D MOL |
|||
| Formula |
C17H20F6N2O3
|
||||
| InChI |
InChI=1S/C17H20F6N2O3/c18-16(19,20)9-27-12-4-5-14(28-10-17(21,22)23)13(7-12)15(26)25-8-11-3-1-2-6-24-11/h4-5,7,11,24H,1-3,6,8-10H2,(H,25,26)
|
||||
| InChIKey |
DJBNUMBKLMJRSA-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 54143-55-4
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9214, 534983, 6136451, 7979229, 8152135, 11120255, 11120743, 11121231, 11121382, 11121862, 11362451, 11365013, 11367575, 11370173, 11370174, 11373176, 11375737, 11466763, 11467883, 11486365, 14879920, 26752195, 26752196, 29222491, 46508078, 47216560, 47216561, 47440010, 47515100, 47588785, 47736232, 47810528, 47959497, 48258984, 48416013, 49699155, 49877342, 50104858, 50104859, 50484619, 53787709, 57321748, 85202322, 85787715, 85788502, 92308773, 92309733, 92714655, 96024657, 99300647
|
||||
| SuperDrug ATC ID |
C01BC04
|
||||
| SuperDrug CAS ID |
cas=054143554
|
||||
| Target and Pathway | |||||
| Target(s) | E3 ubiquitin protein ligase COP1 | Target Info | Modulator | [556264] | |
| KEGG Pathway | p53 signaling pathway | ||||
| Ubiquitin mediated proteolysis | |||||
| PANTHER Pathway | P53 pathway feedback loops 1 | ||||
| References | |||||
| Ref 538298 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 075442. | ||||
| Ref 539654 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2560). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

