Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D03PIO
|
||||
| Former ID |
DNC010245
|
||||
| Drug Name |
IODORESINIFERATOXIN
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [529209] | ||
| Structure |
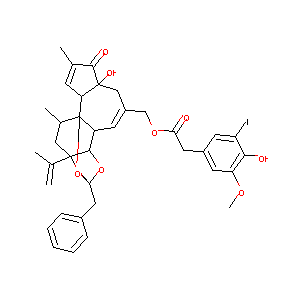
|
Download2D MOL |
|||
| Formula |
C37H39IO9
|
||||
| Canonical SMILES |
CC1CC2(C3C4C1(C5C=C(C(=O)C5(CC(=C4)COC(=O)CC6=CC(=C(C(=<br />C6)I)O)OC)O)C)OC(O3)(O2)CC7=CC=CC=C7)C(=C)C
|
||||
| InChI |
1S/C37H39IO9/c1-20(2)35-16-22(4)37-26(33(35)45-36(46-35,47-37)18-23-9-7-6-8-10-23)12-25(17-34(42)29(37)11-21(3)32(34)41)19-44-30(39)15-24-13-27(38)31(40)28(14-24)43-5/h6-14,22,26,29,33,40,42H,1,15-19H2,2-5H3/t22-,26+,29-,33-,34-,35-,36-,37-/m1/s1
|
||||
| InChIKey |
TZUJORCXGLGWDV-DZBJMWFRSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Transient receptor potential cation channel subfamily V member 4 | Target Info | Inhibitor | [527001] | |
| Vanilloid receptor 1 | Target Info | Inhibitor | [529209] | ||
| Vanilloid receptor | Target Info | Inhibitor | [529209] | ||
| NetPath Pathway | IL2 Signaling Pathway | ||||
| Pathway Interaction Database | Trk receptor signaling mediated by the MAPK pathway | ||||
| Trk receptor signaling mediated by PI3K and PLC-gamma | |||||
| References | |||||
| Ref 527001 | Bioorg Med Chem Lett. 2004 Apr 5;14(7):1693-6.N-4-methansulfonamidobenzyl-N'-2-substituted-4-tert-butyl-benzyl thioureas as potent vanilloid receptor antagonistic ligands. | ||||
| Ref 529209 | J Med Chem. 2008 Jan 10;51(1):57-67. Epub 2007 Dec 12.Stereospecific high-affinity TRPV1 antagonists: chiral N-(2-benzyl-3-pivaloyloxypropyl) 2-[4-(methylsulfonylamino)phenyl]propionamide analogues. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

