Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0ZD3C
|
||||
| Former ID |
DIB016028
|
||||
| Drug Name |
Gemigliptin
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Diabetes [ICD9: 253.5, 588.1; ICD10:E23.2, N25.1] | Phase 3 | [1] | ||
| Company |
LG Life Sciences Ltd
|
||||
| Structure |
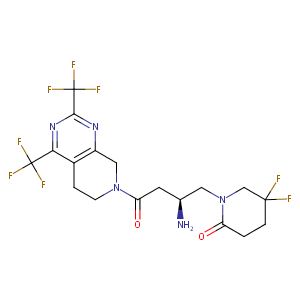
|
Download2D MOL |
|||
| Formula |
C18H19F8N5O2
|
||||
| Canonical SMILES |
C1CN(Cc2c1c(nc(n2)C(F)(F)F)C(F)(F)F)C(=O)C[C@@H](CN1C(=<br />O)CCC(C1)(F)F)N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Dipeptidyl peptidase IV | Target Info | Inhibitor | [2] | |
| KEGG Pathway | Protein digestion and absorption | ||||
| NetPath Pathway | IL2 Signaling Pathway | ||||
| TGF_beta_Receptor Signaling Pathway | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT01990469) Efficacy and Safety of Gemigliptin 50mg qd Added in Patients With Type 2 Diabetes Inadequately Controlled on Glimepiride and Metformin. U.S. National Institutes of Health. | ||||
| REF 2 | Evaluation of the pharmacokinetics of the DPP-4 inhibitor gemigliptin when coadministered with rosuvastatin or irbesartan to healthy subjects. Curr Med Res Opin. 2015 Feb;31(2):229-41. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

