Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0Z5EM
|
||||
| Former ID |
DAP000437
|
||||
| Drug Name |
Cephalexin
|
||||
| Synonyms |
Adcadina; Ades[prex; Alcephin; Alexin; Alsporin; Ambal; Aristosporin; Azabort; Bactopenor; Beliam; Biocef; CEX; Carnosporin; Cefablan; Cefacet; Cefadal; Cefadin; Cefadina; Cefalekey; Cefaleksin; Cefalessina; Cefalexgobens; Cefalexin; Cefalexina; Cefalexine; Cefalexinum; Cefalin; Cefalival; Cefaloto; Cefaseptin; Cefax; Ceffanex; Cefibacter; Ceflax; Ceforal; Cefovit; Celexin; Cepastar; Cepexin; Cephacillin; Cephalexine; Cephalexinum; Cephalobene; Cephanasten; Cephaxin; Cephin; Cepol; Ceporex; Ceporexin; Ceporexine; Check; Cophalexin; Domucef; Doriman; Durantel; Efemida; Erocetin; Factagard; Felexin; Fexin; Ibilex; Ibrexin; Inphalex; Karilexina; Kefalospes; Keflet; Keflex; Kefolan; Keforal; Keftab; Kekrinal; Kidolex; Lafarine; Larixin; Lenocef; Lexibiotico; Loisine; Lonflex; Lopilexin; Losporal; Madlexin; Maksipor; Mamalexin; Mamlexin; Medolexin; Medoxine; Neokef; Neolexina; Noveol; Novolexin; Nufex; Oracef; Oriphex; Oroxin; Ortisporina; Ospexin; Palitrex; Pectril; Prindex; Pyassan; Rilexine; Roceph; Rogevil; Sanaxin; Sartosona; Sencephalin; Sepexin; Servicef; Servispor; Sialexin; Sinthecillin; Sintolexyn; Sporicef; Sporidex; Syncl; Syncle; Synecl; Tepaxin; Theratrex; Tokiolexin; Uphalexin; Viosporine; Voxxim; Winlex; Zabytrex; Zozarine; Cefalessina [DCIT]; Cefalexin Scand Pharm; Cefalexin Sodium; Cefalexin generics; Cefalexin hydrate; Cefalexin monohydrate; Cefalexina Northia; Cefalexina Richet; Cephalex von ct; Cephalexin hydrate; Cephalexin monohydrate; Ceporex Forte; Durantel DS; Henina Oral; Panixine Disperdose; Roceph Distab; Lilly 66873; S 6437; SQ 20248; Cefa-iskia; Cefalexin (JP15); Cefalexin.H2O; Cefalexina [INN-Spanish]; Cefalexine [INN-French]; Cefalexinum [INN-Latin]; Cephalexin(USP); Cephalexin (anhydrous); Cephalexin 1-hydrate; Cephalexin 1-wasser; Cephalexin [USAN:BAN]; Cephalexin.H2O; Ceporexin-E; Cusisporina-Cefalox; Ed A-Ceph; KS-1134; Keflex (TN); Keftab (TN); L-Keflex; Panixine disperdose (TN); Sporidex (TN); Cephalexin, (6R-(6alpha,7beta))-Isomer; 7-(D-2-Amino-2-phenylacetamido)-3-methyl-delta (sup 3)-cephem-4-carboxylic acid; 7-(D-2-Amino-2-phenylacetamido)-3-methyl-delta3-cephem-4-carboxylic acid; 7-(D-alpha-Aminophenylacetamido)desacetoxycephalosporanic acid
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antibiotics
|
||||
| Company |
Eli Lilly
|
||||
| Structure |
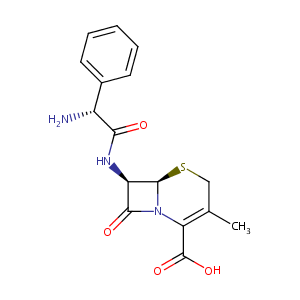
|
Download2D MOL |
|||
| Formula |
C16H17N3O4S
|
||||
| InChI |
InChI=1S/C16H17N3O4S/c1-8-7-24-15-11(14(21)19(15)12(8)16(22)23)18-13(20)10(17)9-5-3-2-4-6-9/h2-6,10-11,15H,7,17H2,1H3,(H,18,20)(H,22,23)/t10-,11-,15-/m1/s1
|
||||
| InChIKey |
ZAIPMKNFIOOWCQ-UEKVPHQBSA-N
|
||||
| CAS Number |
CAS 15686-71-2
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9112, 594576, 625390, 7847329, 7978893, 8169783, 11466386, 11467506, 11486112, 14778344, 14876054, 26719711, 29215501, 34669971, 46386774, 46506749, 47291384, 47515590, 47811031, 48259499, 48415740, 48631157, 49698453, 50050891, 50064641, 50109863, 56314309, 57310443, 79988419, 85788571, 87246423, 92308234, 103682056, 104029668, 104300019, 108667050, 121277919, 124658838, 124766007, 126672951, 129874110, 134221931, 134337733, 134991978, 135692166, 136367997, 137001569, 137240151, 139157607, 144089066
|
||||
| SuperDrug ATC ID |
J01DB01
|
||||
| SuperDrug CAS ID |
cas=015686712
|
||||
| Target and Pathway | |||||
| Target(s) | DNA | Target Info | Binder | [535908] | |
| References | |||||
| Ref 468065 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4832). | ||||
| Ref 538201 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 061969. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

