Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0Y8BW
|
||||
| Former ID |
DIB008456
|
||||
| Drug Name |
TMC-647055
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | HCV infection [ICD9: 070.4, 070.5, 070.70; ICD10:B17.1, B18.2] | Phase 1 | [523173] | ||
| Company |
Janssen research & development
|
||||
| Structure |
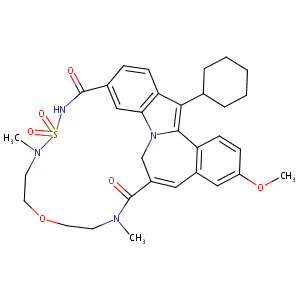
|
Download2D MOL |
|||
| Formula |
C32H38N4O6S
|
||||
| Canonical SMILES |
CN1CCOCCN(S(=O)(=O)NC(=O)C2=CC3=C(C=C2)C(=C4N3CC(=CC5=C<br />4C=CC(=C5)OC)C1=O)C6CCCCC6)C
|
||||
| InChI |
1S/C32H38N4O6S/c1-34-13-15-42-16-14-35(2)43(39,40)33-31(37)22-9-11-27-28(19-22)36-20-24(32(34)38)17-23-18-25(41-3)10-12-26(23)30(36)29(27)21-7-5-4-6-8-21/h9-12,17-19,21H,4-8,13-16,20H2,1-3H3,(H,33,37)
|
||||
| InChIKey |
UOBYJVFBFSLCTQ-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| Target and Pathway | |||||
| Target(s) | Hepatitis C virus NS5B polymerase | Target Info | Inhibitor | [550180] | |
| References | |||||
| Ref 523173 | ClinicalTrials.gov (NCT01202825) TMC647055HPC1001 - First-in-human Trial to Examine Safety, Tolerability and Pharmacokinetics (How the Drug is Absorbed Into the Bloodstream) of Increasing Single OralDoses and of Increasing Repeated Oral Doses of TMC647055 in Healthy Volunteers and in Hepatitis C Virus Infected Patients. U.S. National Institutes of Health. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

