Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0W2AN
|
||||
| Former ID |
DIB004669
|
||||
| Drug Name |
Emepronium
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Urinary incontinence [ICD9: 788.3; ICD10:N39.3, N39.4, R32] | Approved | [551871] | ||
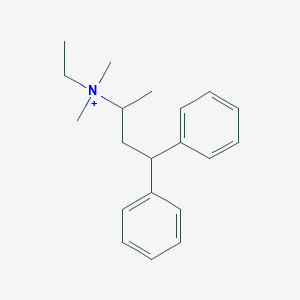
| Structure |
|
Download2D MOL |
|||
| Formula |
C20H28BrN
|
||||
| Canonical SMILES |
CC[N+](C)(C)C(C)CC(c1ccccc1)c2ccccc2
|
||||
| InChI |
1S/C20H28N/c1-5-21(3,4)17(2)16-20(18-12-8-6-9-13-18)19-14-10-7-11-15-19/h6-15,17,20H,5,16H2,1-4H3/q+1
|
||||
| InChIKey |
JEJBJBKVPOWOQK-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Acetylcholine receptor | Target Info | Modulator | [533659], [551871] | |
| KEGG Pathway | Neuroactive ligand-receptor interaction | ||||
| References | |||||
| Ref 533659 | Classification of the presynaptic muscarinic receptor subtype that regulates 3H-acetylcholine secretion in the guinea pig urinary bladder in vitro. J Pharmacol Exp Ther. 1995 Jul;274(1):458-68. | ||||
| Ref 551871 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

