Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0V9WF
|
||||
| Former ID |
DAP001395
|
||||
| Drug Name |
Lestaurtinib
|
||||
| Synonyms |
A 1544750; CEP 701; KT 5555; KT5555; SP 924; CEP-701; KT-5555; SPM-924; Lestaurtinib (USAN/INN)
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Acute myeloid leukemia [ICD9: 205; ICD10:C92.0] | Approved (orphan drug) | [537117], [541016] | ||
| Myeloid leukemia [ICD9: 208.9; ICD10:C92] | Phase 3 | [537117], [541016] | |||
| Psoriasis [ICD9: 696; ICD10:L40] | Phase 2/3 | [528330], [529136], [541016] | |||
| Acute myeloid leukemia; Prostate cancer; Pancreatic cancer [ICD9: 140-199, 140-229, 157, 205.0, 210-229; ICD10:C25, C92.0, D10-D36, D3A] | Phase 2 | [537117], [541016] | |||
| Therapeutic Class |
Anticancer Agents
|
||||
| Company |
Cephalon; Kyowa Hakko Kogyo
|
||||
| Structure |
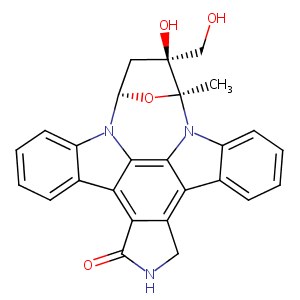
|
Download2D MOL |
|||
| Formula |
C26H21N3O4
|
||||
| InChI |
InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1
|
||||
| InChIKey |
UIARLYUEJFELEN-LROUJFHJSA-N
|
||||
| CAS Number |
CAS 111358-88-4
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
14881395, 26683764, 29306097, 47206529, 50113274, 51950713, 57341827, 71821285, 91614175, 92721422, 103722924, 104162421, 124893551, 124893552, 124893553, 126619159, 126644891, 134338603, 135083872, 135698729, 137237684, 137267229, 141704182, 144206186, 162012051, 162187603, 163125761, 164228175, 164761562, 172087004, 177749083, 178102300, 179149589, 198961101, 204370206, 223654877, 227838931, 241376436, 248732980, 249815159, 252157256, 252215690
|
||||
| Target and Pathway | |||||
| Target(s) | FL cytokine receptor | Target Info | Modulator | [528330], [529136] | |
| References | |||||
| Ref 528330 | A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006 Nov 15;108(10):3262-70. Epub 2006 Jul 20. | ||||
| Ref 529136 | Lestaurtinib (CEP701) is a JAK2 inhibitor that suppresses JAK2/STAT5 signaling and the proliferation of primary erythroid cells from patients with myeloproliferative disorders. Blood. 2008 Jun 15;111(12):5663-71. Epub 2007 Nov 5. | ||||
| Ref 537117 | Emerging drugs for psoriasis. Expert Opin Emerg Drugs. 2009 Mar;14(1):145-63. | ||||
| Ref 541016 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5672). | ||||
| Ref 528330 | A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006 Nov 15;108(10):3262-70. Epub 2006 Jul 20. | ||||
| Ref 529136 | Lestaurtinib (CEP701) is a JAK2 inhibitor that suppresses JAK2/STAT5 signaling and the proliferation of primary erythroid cells from patients with myeloproliferative disorders. Blood. 2008 Jun 15;111(12):5663-71. Epub 2007 Nov 5. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

