Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0V5KW
|
||||
| Former ID |
DIB007563
|
||||
| Drug Name |
EZN-2279
|
||||
| Synonyms |
PEG-rADA; PEGylated recombinant adenosine deaminase (SCID), Sigma-Tau
|
||||
| Indication | Immunodeficiency [ICD9: 279.3; ICD10:D84.9] | Phase 3 | [523593] | ||
| Company |
Enzon Pharmaceuticals Inc
|
||||
| Structure |
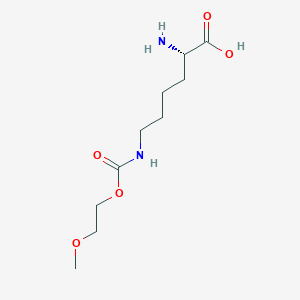
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Adenosine deaminase | Target Info | Modulator | [551689] | |
| NetPath Pathway | TCR Signaling Pathway | ||||
| IL2 Signaling Pathway | |||||
| PANTHER Pathway | Adenine and hypoxanthine salvage pathway | ||||
| PathWhiz Pathway | Purine Metabolism | ||||
| Reactome | Purine salvage | ||||
| WikiPathways | Metabolism of nucleotides | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

