Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0S5UH
|
||||
| Former ID |
DAP000789
|
||||
| Drug Name |
Clofazimine
|
||||
| Synonyms |
CFZ; Chlofazimine; Clofazimina; Clofaziminum; Lampren; Lamprene; B 663; G 30320; SMP2_000339; B 663 (Pharmaceutical); B 663 (VAN); B 663, pharmaceutical; B-663; B. 663; Clofazimina [INN-Spanish]; Clofaziminum [INN-Latin]; G-30320; Lamprene (TN); Liposome-encapsulated clofazimine; Clofazimine [USAN:INN:BAN]; G-30,320; Clofazimine (JAN/USP/INN); N,5-bis(4-chlorophenyl)-3-propan-2-yliminophenazin-2-amine; N,5-Bis(4-chlorophenyl)-3,5-dihydro-3-(isopropylimino)phenazin-2-amine; N,5-Bis(4-chlorophenyl)-3,5-dihydro-3-(1-methylethyl)imino)-2-phenazinamine; N,5-Bis(4-chlorophenyl)-3,5-dihydro-3-[(1-methylethyl)imino]-2-phenazinamine; N,5-bis(4-chlorophenyl)-3-(propan-2-ylimino)-3,5-dihydrophenazin-2-amine; (3Z)-N,5-bis(4-chlorophenyl)-3-[(1-methylethyl)imino]-3,5-dihydrophenazin-2-amine; 3-(p-Chloranilino)-10-(p-chlorophenyl)-2,10-dihydro-2-(isopropylimino)-phenazine; 3-(p-Chloranilino)-10-(p-chlorophenyl)-2,10-dihydro-2-(isopropylimino)phenazine; 3-(p-Chloranilino)-10-(p-chlorphenyl)-2,10-dihydro-2-(isopropylimino)-phenazin; 3-(p-Chloranilino)-10-(p-chlorphenyl)-2,10-dihydro-2-(isopropylimino)-phenazin [German]; 3-(p-Chloroanilino)-10-(p-chlorophenyl)-2,10-dihydro-2-(isopropylimino)phenazine
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Crohn's disease [ICD9: 555; ICD10:K50] | Approved | [538526] | ||
| Therapeutic Class |
Antiinflammatory Agents
|
||||
| Company |
Norvatis Phamaceuticals Corporation
|
||||
| Structure |
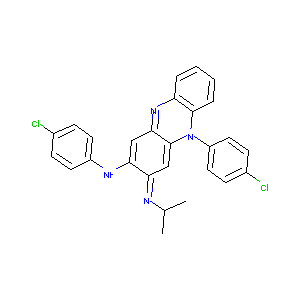
|
Download2D MOL |
|||
| Formula |
C27H22Cl2N4
|
||||
| Canonical SMILES |
CC(C)N=C1C=C2C(=NC3=CC=CC=C3N2C4=CC=C(C=C4)Cl)C=C1NC5=C<br />C=C(C=C5)Cl
|
||||
| InChI |
1S/C27H22Cl2N4/c1-17(2)30-24-16-27-25(15-23(24)31-20-11-7-18(28)8-12-20)32-22-5-3-4-6-26(22)33(27)21-13-9-19(29)10-14-21/h3-17,31H,1-2H3
|
||||
| InChIKey |
WDQPAMHFFCXSNU-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 2030-63-9
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9132, 426821, 602744, 627688, 855829, 4302579, 7847344, 7978968, 8151803, 10321645, 11112506, 11466404, 11467524, 11486188, 14761008, 15356285, 24714740, 24893114, 29221949, 32963436, 46386952, 46508174, 47275676, 47349472, 47499671, 47795160, 48243512, 48415799, 49681815, 49698463, 50004010, 50100467, 50124245, 50376246, 56314325, 57321463, 92125497, 92308020, 92308564, 93167165, 93619701, 96079551, 103400985, 103755939, 103914133, 104153270, 104301631, 118816613, 121269827, 121363168
|
||||
| SuperDrug ATC ID |
J04BA01
|
||||
| SuperDrug CAS ID |
cas=002030639
|
||||
| Target and Pathway | |||||
| Target(s) | Bacterial DNA | Target Info | Modulator | ||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

