Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0Q7UD
|
||||
| Former ID |
DNC008718
|
||||
| Drug Name |
ABT-888
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
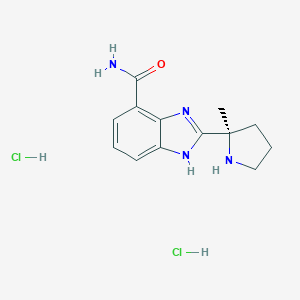
|
Download2D MOL |
|||
| Formula |
C13H16N4O
|
||||
| InChI |
InChI=1S/C13H16N4O/c1-13(6-3-7-15-13)12-16-9-5-2-4-8(11(14)18)10(9)17-12/h2,4-5,15H,3,6-7H2,1H3,(H2,14,18)(H,16,17)/t13-/m1/s1
|
||||
| InChIKey |
JNAHVYVRKWKWKQ-CYBMUJFWSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
17418828, 23919521, 36457782, 48431542, 53786540, 57298845, 78845658, 85742017, 93581011, 99432361, 99443703, 103628124, 123051081, 124490432, 124756927, 125163734, 126522682, 126626210, 126661310, 126665801, 126731194, 131271125, 131465098, 134223378, 134345923, 135251923, 136367452, 137275905, 137517732, 138192110, 152090859, 152258095, 152344520, 160646934, 160968315, 162011690, 162202719, 163776182, 163907937, 164041817, 164194024, 164339419, 174531428, 178103989, 184816212, 194943753, 198953538, 198994026, 208265497, 212343015
|
||||
| Target and Pathway | |||||
| Target(s) | Poly ADP ribose polymerase (PARP) | Target Info | Modulator | [1572591] | |
| References | |||||
| Ref 524798 | ClinicalTrials.gov (NCT02163694) A Phase 3 Randomized, Placebo-controlled Trial of Carboplatin and Paclitaxel With or Without Veliparib (ABT-888) in HER2-negative Metastatic or Locally Advanced Unresectable BRCA-associated Breast Cancer. U.S. National Institutes of Health. | ||||
| Ref 542441 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7417). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

