Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0KX9B
|
||||
| Former ID |
DNCL002804
|
||||
| Drug Name |
EP-101
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Chronic obstructive pulmonary disease [ICD9: 490-492, 494-496; ICD10:J40-J44, J47] | Phase 2 | [523605] | ||
| Company |
Elevation Pharmaceuticals
|
||||
| Structure |
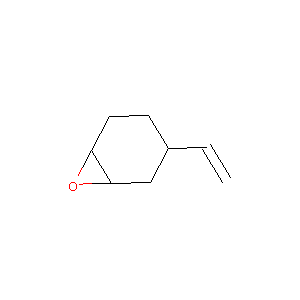
|
Download2D MOL |
|||
| Formula |
C8H12O
|
||||
| Canonical SMILES |
C=CC1CCC2C(C1)O2
|
||||
| InChI |
1S/C8H12O/c1-2-6-3-4-7-8(5-6)9-7/h2,6-8H,1,3-5H2
|
||||
| InChIKey |
SLJFKNONPLNAPF-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
92519, 5964499, 8155380, 15321303, 24849235, 29226618, 49999767, 56367848, 57324518, 80447510, 88835268, 104316107, 117394656, 117394657, 121278413, 125643625, 126564565, 126676587, 128317810, 129253052, 131293880, 134972080, 137181223, 142310814, 143490727, 152041149, 152235330, 162096454, 163306405, 163415435, 163803152, 164791602, 165374121, 172089146, 172886474, 176325026, 184586929, 204425894, 210024047, 223653908, 223773569, 226419812, 249852374, 250216422, 252032301, 252404318, 252465773
|
||||
| Target and Pathway | |||||
| Target(s) | Muscarinic receptor | Target Info | Antagonist | [532169] | |
| PathWhiz Pathway | Muscle/Heart Contraction | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

