Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0K9MY
|
||||
| Former ID |
DCL000639
|
||||
| Drug Name |
Saxagliptin
|
||||
| Synonyms |
Onglyza; BMS 477118-11; BMS-477118; Kombiglyze XR (TN); OPC-262; Onglyza (TN); BMS-477118-11; (1S,5S)-2-[2-amino-2-(3-hydroxy-1-adamantyl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile; 2-Azabicyclo[3.1.0]hexane-3-carbonitrile, 2-[(2S)-amino(3-hydroxytricyclo[3.3
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Type 2 diabetes [ICD9: 250; ICD10:E11] | Approved | [1], [2] | ||
| Company |
Bristol-Myers Squibb; AstraZeneca
|
||||
| Structure |
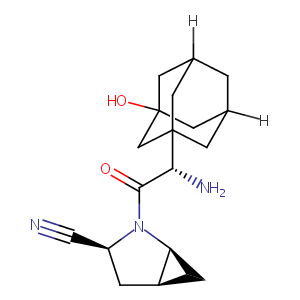
|
Download2D MOL |
|||
| Formula |
C18H25N3O2
|
||||
| InChI |
InChI=1S/C18H25N3O2/c19-8-13-2-12-3-14(12)21(13)16(22)15(20)17-4-10-1-11(5-17)7-18(23,6-10)9-17/h10-15,23H,1-7,9,20H2/t10?,11?,12-,13+,14+,15-,17?,18?/m1/s1
|
||||
| InChIKey |
QGJUIPDUBHWZPV-SGTAVMJGSA-N
|
||||
| CAS Number |
CAS 361442-04-8
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
16328544, 16675586, 23422497, 42323525, 46512075, 57304394, 75512904, 92722527, 96025679, 99443245, 103500900, 104096574, 104253199, 121147387, 121362351, 123051077, 124758670, 124772084, 126618190, 126644889, 136340362, 137251549, 139567127, 144076400, 144206461, 152035966, 160655903, 160967860, 162011574, 162164961, 175267813, 175610961, 178102934, 184811995, 223668663, 223705237, 224543974, 225361148, 226407286, 246280642, 251970941, 252154978, 252447400
|
||||
| SuperDrug ATC ID |
A10BH03
|
||||
| Target and Pathway | |||||
| Target(s) | Dipeptidyl peptidase IV | Target Info | Inhibitor | [1], [3], [4] | |
| KEGG Pathway | Protein digestion and absorption | ||||
| NetPath Pathway | IL2 Signaling Pathway | ||||
| TGF_beta_Receptor Signaling Pathway | |||||
| References | |||||
| REF 1 | Hughes B: 2009 FDA drug approvals. Nat Rev Drug Discov. 2010 Feb;9(2):89-92. | ||||
| REF 2 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6316). | ||||
| REF 3 | Emerging drug candidates of dipeptidyl peptidase IV (DPP IV) inhibitor class for the treatment of Type 2 Diabetes. Curr Drug Targets. 2009 Jan;10(1):71-87. | ||||
| REF 4 | Clinical pipeline report, company report or official report of AstraZeneca (2009). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

