Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0H3WI
|
||||
| Former ID |
DAP000173
|
||||
| Drug Name |
Ribavirin
|
||||
| Synonyms |
Copegus; Cotronak; RBV; RTC; RTCA; Ravanex; Rebetol; Rebetron; Rebretron; Ribacine; Ribamide; Ribamidil; Ribamidyl; Ribasphere; Ribav; Ribavirina; Ribavirine; Ribavirinum; Ribovirin; Tribavirin; Varazid; Vilona; Viramid; Viramide; Virazid; Virazide; Virazole; Ribavirin Capsules; R 9644; SCH 18908; C-Virin; Copegus (TN); DRG-0028; Drug: Ribavirin; ICN-1229; KS-1104; R-964; RG-964; Rebetol (TN); Ribasphere (TN); Ribavirin [USAN:INN]; Ribavirina [INN-Spanish]; Ribavirine [INN-French]; Ribavirinum [INN-Latin]; Vilona (TN); Virazole (Ribavirin) Inhalation Solution; Virazole (TN); AA-504/07617051; Ro 20-9963/000; Ro-20-9963; Ribavirin (JAN/USP/INN); 1-.beta.-D-Ribofuranosyl-1,2,4-triazolo-3-carboxamide; 1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,2,4-triazole-3-carboxamide; 1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1H-1,2,4-triazole-3-carboxamide; 1-beta-D-Ribofuranosyl-1,2,4-triazole-3-carboxamide; 1-beta-D-Ribofuranosyl-1H-1,2,4-triazole-3-carboxamide; 1-beta-D-ribofuranosyl-1-H-1,2,4-triazole-3-carboxamide
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antiviral Agents
|
||||
| Company |
Hoffmann-La Roche pharmaceutical company
|
||||
| Structure |
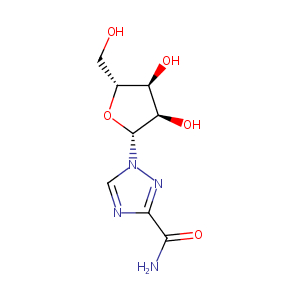
|
Download2D MOL |
|||
| Formula |
C8H12N4O5
|
||||
| InChI |
InChI=1S/C8H12N4O5/c9-6(16)7-10-2-12(11-7)8-5(15)4(14)3(1-13)17-8/h2-5,8,13-15H,1H2,(H2,9,16)/t3-,4-,5-,8-/m1/s1
|
||||
| InChIKey |
IWUCXVSUMQZMFG-AFCXAGJDSA-N
|
||||
| CAS Number |
CAS 36791-04-5
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
595938, 7847489, 7980513, 8139972, 8150084, 8175073, 11335957, 11361196, 11364740, 11367302, 11369864, 11372951, 11374942, 11378029, 11462168, 11491691, 11493066, 11495640, 11528313, 12059582, 14798456, 15122005, 17389529, 17405619, 24278685, 25621753, 26538475, 26612558, 26679740, 34678832, 46505883, 47291173, 47365228, 47440296, 47515344, 47589035, 47885447, 47885448, 48035157, 48416516, 48424047, 48425596, 48631153, 49699015, 50105410, 50105411, 50105412, 50105413, 50474786, 53778169
|
||||
| SuperDrug ATC ID |
J05AB04
|
||||
| SuperDrug CAS ID |
cas=036791045
|
||||
| Drug Resistance Mutation (DRM) | |||||
| DRM | DRM Info | ||||
| Target and Pathway | |||||
| Target(s) | Inosine-5'-monophosphate dehydrogenase | Target Info | Inhibitor | [536140], [536154] | |
| References | |||||
| Ref 538312 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 076192. | ||||
| Ref 541922 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6842). | ||||
| Ref 536140 | A randomized, double-blind, placebo-controlled dose-escalation trial of merimepodib (VX-497) and interferon-alpha in previously untreated patients with chronic hepatitis C. Antivir Ther. 2005;10(5):635-43. | ||||
| Ref 536154 | Treating HCV with ribavirin analogues and ribavirin-like molecules. J Antimicrob Chemother. 2006 Jan;57(1):8-13. Epub 2005 Nov 17. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

