Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0H3JF
|
||||
| Former ID |
DNCL002201
|
||||
| Drug Name |
VTX-2337
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Allergy [ICD9: 995.3; ICD10:T78.4] | Discontinued in Phase 2 | [524026] | ||
| Company |
VentiRx Pharmaceuticals
|
||||
| Structure |
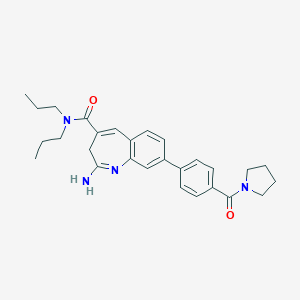
|
Download2D MOL |
|||
| Formula |
C28H34N4O2
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Toll-like receptor 8 | Target Info | Modulator | [550276] | |
| KEGG Pathway | Toll-like receptor signaling pathway | ||||
| NetPath Pathway | Leptin Signaling Pathway | ||||
| TCR Signaling Pathway | |||||
| PANTHER Pathway | Toll receptor signaling pathway | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

