Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0F5BL
|
||||
| Former ID |
DIB015756
|
||||
| Drug Name |
EXO-230
|
||||
| Synonyms |
GLY-230
|
||||
| Indication | Diabetic neuropathy [ICD9: 250, 250.6, 356.0, 356.8; ICD10:E11.40] | Phase 1/2 | [1] | ||
| Company |
Glycadia Inc
|
||||
| Structure |
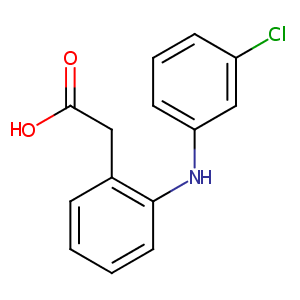
|
Download2D MOL |
|||
| Canonical SMILES |
N(c1c(CC(=O)O)cccc1)c1cc(Cl)ccc1
|
||||
| Target and Pathway | |||||
| Target(s) | Prostaglandin G/H synthase 1 | Target Info | Agonist | [2] | |
| BioCyc Pathway | C20 prostanoid biosynthesis | ||||
| KEGG Pathway | Arachidonic acid metabolism | ||||
| Metabolic pathways | |||||
| Platelet activation | |||||
| Serotonergic synapse | |||||
| NetPath Pathway | TGF_beta_Receptor Signaling Pathway | ||||
| PANTHER Pathway | Inflammation mediated by chemokine and cytokine signaling pathway | ||||
| PathWhiz Pathway | Arachidonic Acid Metabolism | ||||
| WikiPathways | Prostaglandin Synthesis and Regulation | ||||
| Arachidonic acid metabolism | |||||
| Phase 1 - Functionalization of compounds | |||||
| Eicosanoid Synthesis | |||||
| Selenium Micronutrient Network | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT00544934) Multiple Dose Trial of Anti-glycation Agent GLY-230 in Healthy and Diabetic Subjects. U.S. National Institutes of Health. | ||||
| REF 2 | Inhibiting Amadori-modified albumin formation improves biomarkers of podocyte damage in diabetic rats. Physiol Rep. 2013 September; 1(4): e00083. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

