Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0CT9Y
|
||||
| Former ID |
DAP001013
|
||||
| Drug Name |
Clomifene
|
||||
| Synonyms |
Androxal; Chlomaphene; Chloramifene; Cisclomifenum; Cisclomiphene; Clomifen; Clomifeno; Clomifenum; Clomifert; Clomiphene; Clostilbegit; Enclomifene; Enclomifeno; Enclomifenum; Enclomiphen; Enclomiphene; Klostilbegit; Transclomifenum; Transclomiphene; Zuclomifene; Zuclomifeno; Zuclomifenum; Zuclomiphene; Clomiphene B;Enclomiphene [USAN]; ISOMER B; Zuclomiphene [USAN]; Cis-Clomifene; Cis-Clomiphene; Clomid (TN); Clomifene (INN); Clomifene (TN); Clomifene [INN:BAN]; Clomifeno [INN-Spanish]; Clomifenum [INN-Latin]; En-Clomiphene; Enclomifeno [INN-Spanish]; Enclomifenum [INN-Latin]; Enclomiphene (USAN); Milophene (TN); RMI 16,289; RMI-16289; RMI-16312; Serophene (TN); Trans-Clomifene; Trans-Clomiphene; Zuclomifeno [INN-Spanish]; Zuclomifenum [INN-Latin]; Zuclomiphene (USAN); Cis-2-(p-(2-Chloro-1,2-diphenylvinyl)phenoxy)triethylamine; Trans-2-(p-(2-Chloro-1,2-diphenylvinyl)phenoxy)triethylamine; Cis-2-(4-(2-Chloro-1,2-diphenylethenyl)phenoxy)-N,N-diethylethanamine; Trans-2-(4-(2-Chloro-1,2-diphenylethenyl)phenoxy)-N,N-diethylethanamine; (E)-2-(p-(2-Chloro-1,2-diphenylvinyl)phenoxy)triethylamine; (Z)-2-(p-(2-Chloro-1,2-diphenylvinyl)phenoxy)triethylamine; (Z)-isomer; 1-(p-(beta-Diethylaminoethoxy)-phenyl)-1,2-diphenylchloroethylene; 2-(4-(2-Chloro-1,2-diphenylethenyl)phenoxy)-N,N-diethylethanamine; 2-(p-(2-Chloro-1,2-diphenylvinyl)phenoxy)triethylamine; 2-(p-(beta-Chloro-alpha-phenylstyryl)phenoxy)-triethylamine; 2-(p-(beta-chloro-alpha-phenylstyryl)phenoxy)triethylamine; 2-({4-[(Z)-2-chloro-1,2-diphenylethenyl]phenyl}oxy)-N,N-diethylethanamine; 2-[4-(2-chloro-1,2-diphenylethenyl)phenoxy]-N,N-diethylethanamine; 2-[4-[(E)-2-chloro-1,2-diphenylethenyl]phenoxy]-N,N-diethylethanamine; 2-[4-[(Z)-2-chloro-1,2-diphenylethenyl]phenoxy]-N,N-diethylethanamine; 2-{4-[(E)-2-chloro-1,2-diphenylethenyl]phenoxy}-N,N-diethylethanamine
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Fertility Agents
|
||||
| Structure |
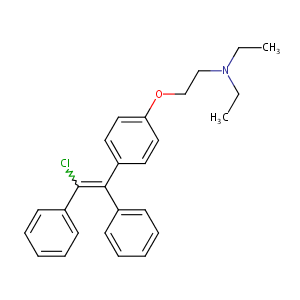
|
Download2D MOL |
|||
| Formula |
C26H28ClNO
|
||||
| InChI |
InChI=1S/C26H28ClNO/c1-3-28(4-2)19-20-29-24-17-15-22(16-18-24)25(21-11-7-5-8-12-21)26(27)23-13-9-6-10-14-23/h5-18H,3-4,19-20H2,1-2H3/b26-25+
|
||||
| InChIKey |
GKIRPKYJQBWNGO-OCEACIFDSA-N
|
||||
| CAS Number |
CAS 911-45-5
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9134, 7978971, 7979159, 8653308, 14879465, 32248894, 46504463, 47646665, 48094648, 48169503, 48169504, 49698470, 50058156, 50085975, 50124218, 51092022, 56352921, 57409058, 75735317, 81093254, 85788573, 90452382, 92309293, 93165675, 93166374, 96025593, 103249489, 104057024, 110525086, 117721819, 124800080, 124893612, 126690411, 131742069, 135024045, 139650876, 164235770, 175270141, 176484319, 179148468, 184022027, 198962245, 223365954, 224924849, 250133940, 251960682, 252635354
|
||||
| ChEBI ID |
ChEBI:3752
|
||||
| SuperDrug ATC ID |
G03GB02
|
||||
| SuperDrug CAS ID |
cas=000911455
|
||||
| Target and Pathway | |||||
| Target(s) | Estrogen receptor | Target Info | Modulator | [536404] | |
| Pathway Interaction Database | Regulation of nuclear SMAD2/3 signaling | ||||
| Signaling events mediated by HDAC Class II | |||||
| Plasma membrane estrogen receptor signaling | |||||
| LKB1 signaling events | |||||
| Regulation of Telomerase | |||||
| ATF-2 transcription factor network | |||||
| AP-1 transcription factor network | |||||
| FOXM1 transcription factor network | |||||
| Validated nuclear estrogen receptor alpha network | |||||
| Signaling mediated by p38-alpha and p38-beta | |||||
| FOXA1 transcription factor network | |||||
| References | |||||
| Ref 536296 | Emerging drugs for hypogonadism. Expert Opin Emerg Drugs. 2006 Nov;11(4):685-707. | ||||
| Ref 542607 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7619). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

