Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D08TQP
|
||||
| Former ID |
DIB011725
|
||||
| Drug Name |
INX-189
|
||||
| Synonyms |
INX-08032; INX-08189; INX-108; Nucleoside polymerase inhibitors (HCV infection), Cardiff University/Rega Institute; Nucleoside polymerase inhibitors (oral, HCV infection), Inhibitex
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | HCV infection [ICD9: 070.4, 070.5, 070.70; ICD10:B17.1, B18.2] | Phase 2 | [523604] | ||
| Company |
Cardiff University
|
||||
| Structure |
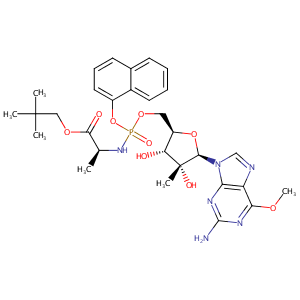
|
Download2D MOL |
|||
| Formula |
C30H39N6O9P
|
||||
| Canonical SMILES |
CC(C)(COC(=O)[C@@H](NP(=O)(Oc1cccc2c1cccc2)OC[C@H]1O[C@<br />H]([C@]([C@@H]1O)(C)O)n1cnc2c1nc(nc2OC)N)C)C
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Hepatitis C virus NS5B polymerase | Target Info | Inhibitor | [550009] | |
| References | |||||
| Ref 523604 | ClinicalTrials.gov (NCT01425970) Chronically-infected HCV Genotype 2 and 3 Treatment-naive Subjects: Part A: Safety and Efficacy of INX-08189 With Peg IFN Alfa-2a and Ribavirin. Part B: INX-08189 in Interferon Free Treatment With Daclatasvir and/or Ribavirin. U.S. National Institutes of Health. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

