Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D08DFX
|
||||
| Former ID |
DCL001169
|
||||
| Drug Name |
Canagliflozin
|
||||
| Drug Type |
Small molecular drug
|
||||
| Company |
Johnson & Johnson
|
||||
| Structure |
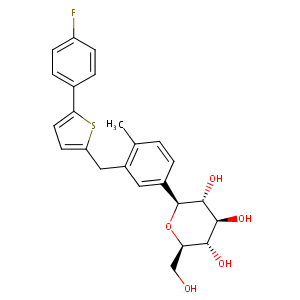
|
Download2D MOL |
|||
| Formula |
C24H25FO5S
|
||||
| InChI |
InChI=1S/C24H25FO5S/c1-13-2-3-15(24-23(29)22(28)21(27)19(12-26)30-24)10-16(13)11-18-8-9-20(31-18)14-4-6-17(25)7-5-14/h2-10,19,21-24,26-29H,11-12H2,1H3/t19-,21-,22+,23-,24+/m1/s1
|
||||
| InChIKey |
XTNGUQKDFGDXSJ-ZXGKGEBGSA-N
|
||||
| CAS Number |
CAS 842133-18-0
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
49811874, 57127075, 125299338, 134358471, 135267033, 136023439, 136340353, 136367529, 136368011, 141631822, 144115779, 144224573, 152159581, 152258221, 160647057, 160695863, 160865968, 162011557, 162169318, 162205127, 164837687, 170500828, 172918748, 174530759, 175266021, 175427146, 178101303, 185964359, 196373210, 198993774, 202567714, 211535181, 223258907, 223471388, 223617458, 223705166, 224378424, 226521104, 248537044, 249737173, 250163176, 252109951, 252215091
|
||||
| SuperDrug ATC ID |
A10BX11
|
||||
| Target and Pathway | |||||
| Target(s) | SLGT2 | Target Info | Modulator | [532651], [532916] | |
| References | |||||
| Ref 467821 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4582). | ||||
| Ref 532651 | 2013 FDA drug approvals. Nat Rev Drug Discov. 2014 Feb;13(2):85-9. | ||||
| Ref 532916 | Canagliflozin, a sodium glucose co-transporter 2 inhibitor, reduces post-meal glucose excursion in patients with type 2 diabetes by a non-renal mechanism: results of a randomized trial. Metabolism. 2014 Oct;63(10):1296-303. | ||||
| Ref 551060 | Clinical pipeline report, company report or official report of Johnson & Johnson (2011). | ||||
| Ref 532651 | 2013 FDA drug approvals. Nat Rev Drug Discov. 2014 Feb;13(2):85-9. | ||||
| Ref 532916 | Canagliflozin, a sodium glucose co-transporter 2 inhibitor, reduces post-meal glucose excursion in patients with type 2 diabetes by a non-renal mechanism: results of a randomized trial. Metabolism. 2014 Oct;63(10):1296-303. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

