Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D07XSN
|
||||
| Former ID |
DAP000644
|
||||
| Drug Name |
Cytarabine
|
||||
| Synonyms |
Alexan; AraC; Arabinocytidine; Arabinofuranosylcytosine; Arabinosylcytosine; Arabitin; Aracytidine; Aracytin; Aracytine; Arafcyt; Citarabina; Cytarabin; Cytarabina; Cytarabinoside; Cytarabinum; Cytarbel; Cytonal; Cytosar; Cytosinearabinoside; DepoCyte; Depocyt; Erpalfa; Iretin; Spongocytidine; Tarabine; Udicil; Arabinosyl Cytosine; Cytarabine liposome injection; Cytosine arabinofuranoside; Cytosine arabinose; Cytosine arabinoside; AR3; BTB15125; CHX 3311; U 19920A; Ara-C; Ara-Cytidine; Beta-Ara C; Beta-Arabinosylcytosine; Beta-cytosine arabinoside; Citarabina [INN-Spanish]; Cytarabinum [INN-Latin]; Cytosar-U; Cytosine arabinoside (VAN); Depocyt (TN); Depocyt (liposomal); Intrathecal (injected into the spinal fluid) DepoCyt; U-19920; Beta-D-Arabinosylcytosine; Cytosar-U (TN); Cytosine beta-D-arabinofuranoside; Cytosine beta-D-arabinofuranoside hydrochloride; Cytosine beta-D-arabinoside; Cytosine-beta-arabinoside; Intrathecal cytarabine (also known as ara-C); U-19,920; CYTARABINE (SEE ALSO CYTARABINE HYDROCHLORIDE 69-74-9); Cytarabine (JP15/USP/INN); Cytarabine [USAN:INN:BAN:JAN]; Cytosine 1-beta-D-arabinofuranoside; Cytosine, beta-D-arabinoside; Cytosine-beta-D-arabinofuranoside; Cytosine-1-beta-D-arabinofuranoside; Ara-C, Cytosine Arabinoside, Cytosar-U, Cytarabine; (beta-D-Arabinofuranosyl)cytosine; 1-.beta.-D-arabinofuranosyl-cytosine; 1-Arabinofuranosylcytosine; 1-beta-D-Arabinofaranosylcytosine; 1-beta-D-Arabinofuranosyl-4-amino-2(1H)pyrimidinone; 1-beta-D-Arabinofuranosylcytosine; 1-beta-D-Arabinofuranosylcytosine, Cytosine Arabinoside; 1-beta-D-Arabinosylcytosine; 1beta-Arabinofuranasylcytosine; 1beta-D-Arabinofuranosylcytosine; 1beta-D-Arabinosylcytosine; 2(1H)-Pyrimidinone, 4-amino-1-D-arabinofuranosyl-[CAS]; 4-Amino-1-arabinofuranosyl-2-oxo-1,2-dihydropyrimidin; 4-Amino-1-arabinofuranosyl-2-oxo-1,2-dihydropyrimidin [Czech]; 4-Amino-1-arabinofuranosyl-2-oxo-1,2-dihydropyrimidine; 4-Amino-1-b-D-arabinofuranosyl-2-(1H)-pyrimidinone; 4-Amino-1-beta-D-arabinofuranosyl-2(1H)-pyrimidinon; 4-Amino-1-beta-D-arabinofuranosyl-2(1H)-pyrimidinon [Czech]; 4-Amino-1-beta-D-arabinofuranosyl-2(1H)-pyrimidinone; 4-amino-1-[(2R,3S,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one; 4-amino-1-beta-D-arabinofuranosylpyrimidin-2(1H)-one
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Anticancer Agents
|
||||
| Company |
Upjohn Corporation
|
||||
| Structure |
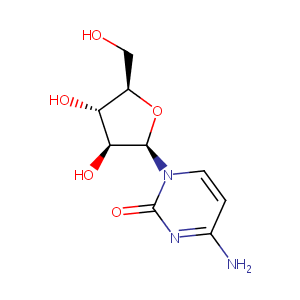
|
Download2D MOL |
|||
| Formula |
C9H13N3O5
|
||||
| InChI |
InChI=1S/C9H13N3O5/c10-5-1-2-12(9(16)11-5)8-7(15)6(14)4(3-13)17-8/h1-2,4,6-8,13-15H,3H2,(H2,10,11,16)/t4-,6-,7+,8-/m1/s1
|
||||
| InChIKey |
UHDGCWIWMRVCDJ-CCXZUQQUSA-N
|
||||
| CAS Number |
CAS 147-94-4
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
5877, 598052, 829245, 3260118, 7847236, 7885952, 7979015, 8153933, 11528303, 11533128, 12146044, 14774115, 15196510, 24858315, 24892435, 26527898, 26719755, 29215144, 29225249, 46386862, 46505879, 47193873, 47573376, 48415837, 49831046, 50105698, 53787662, 56310997, 56311029, 56312269, 56312943, 56313129, 56313612, 76890970, 83110442, 87558791, 90341066, 92308449, 92309014, 93166529, 93167157, 103210760, 103986361, 104253284, 104311532, 117529237, 118048876, 124648720, 124757405, 124799562
|
||||
| ChEBI ID |
ChEBI:28680
|
||||
| SuperDrug ATC ID |
L01BC01
|
||||
| SuperDrug CAS ID |
cas=000147944
|
||||
| Drug Resistance Mutation (DRM) | |||||
| DRM | DRM Info | ||||
| Target and Pathway | |||||
| Target(s) | DNA polymerase | Target Info | Inhibitor | [537233] | |
| References | |||||
| Ref 468059 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4827). | ||||
| Ref 530906 | Incorporation of gemcitabine and cytarabine into DNA by DNA polymerase beta and ligase III/XRCC1. Biochemistry. 2010 Jun 15;49(23):4833-40. | ||||
| Ref 538237 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 071471. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

