Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D07URD
|
||||
| Former ID |
DIB004804
|
||||
| Drug Name |
PRX-102
|
||||
| Synonyms |
Galactosidase enzyme replacement therapy (Fabry disease), Protalix
|
||||
| Indication | Fabry's disease [ICD9: 272.7; ICD10:E75.2] | Phase 1/2 | [1] | ||
| Company |
Protalix biotherapeutics
|
||||
| Structure |
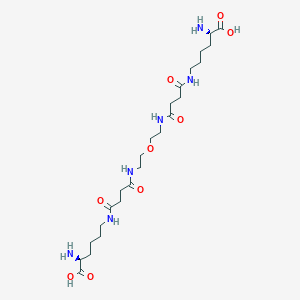
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Alpha-galactosidase A | Target Info | Modulator | [2] | |
| KEGG Pathway | Galactose metabolism | ||||
| Glycerolipid metabolism | |||||
| Sphingolipid metabolism | |||||
| Glycosphingolipid biosynthesis - globo series | |||||
| Lysosome | |||||
| PathWhiz Pathway | Sphingolipid Metabolism | ||||
| Galactose Metabolism | |||||
| Reactome | Glycosphingolipid metabolism | ||||
| WikiPathways | Sphingolipid metabolism | ||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT01981720) Extension Study of PRX-102 for 24 Months. U.S. National Institutes of Health. | ||||
| REF 2 | Characterization of a chemically modified plant cell culture expressed human alpha-Galactosidase-A enzyme for treatment of Fabry disease. Mol Genet Metab. 2015 Feb;114(2):259-67. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

