Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D06VMJ
|
||||
| Former ID |
DCL001113
|
||||
| Drug Name |
Melogliptin
|
||||
| Indication | Type 2 diabetes [ICD9: 250; ICD10:E11] | Phase 2 | [1] | ||
| Company |
Glenmark pharma
|
||||
| Structure |
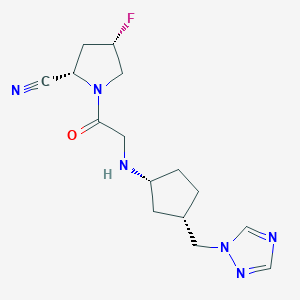
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Dipeptidyl peptidase IV | Target Info | Inhibitor | [2] | |
| KEGG Pathway | Protein digestion and absorption | ||||
| NetPath Pathway | IL2 Signaling Pathway | ||||
| TGF_beta_Receptor Signaling Pathway | |||||
| References | |||||
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800021640) | ||||
| REF 2 | Emerging drug candidates of dipeptidyl peptidase IV (DPP IV) inhibitor class for the treatment of Type 2 Diabetes. Curr Drug Targets. 2009 Jan;10(1):71-87. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

