Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D05UHJ
|
||||
| Former ID |
DIB016307
|
||||
| Drug Name |
SM-10888
|
||||
| Indication | Cognitive disorders [ICD9: 290-294, 294.0, 780.09, 780.9, 780.93; ICD10:F01-F07, F04, F05, R41.3] | Discontinued in Phase 2 | [544850] | ||
| Company |
Sumitomo Pharmaceuticals Co Ltd
|
||||
| Structure |
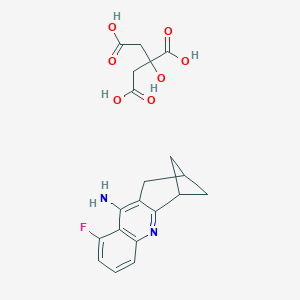
|
Download2D MOL |
|||
| Formula |
C54H55F3N6O14
|
||||
| Canonical SMILES |
c12c(nc3c(c1N)c(F)ccc3)C1CC(C2)C1.C(CC(=O)O)(CC(=O)O)(C<br />(=O)O)O
|
||||
| CAS Number |
CAS 116208-23-2
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Acetylcholinesterase | Target Info | Inhibitor | [532426] | |
| KEGG Pathway | Glycerophospholipid metabolism | ||||
| Cholinergic synapse | |||||
| Pathway Interaction Database | ATF-2 transcription factor network | ||||
| PathWhiz Pathway | Phospholipid Biosynthesis | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

