Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D05UBX
|
||||
| Former ID |
DAP001179
|
||||
| Drug Name |
Cefmetazole
|
||||
| Synonyms |
CMZ; Cefmetazolo; Cefmetazolum; Cefmetazole Monosodium Salt; CS 1170; SKF 83088; U 72791; CS-1170; Cefmetazole [USAN:INN]; Cefmetazolo [INN-Spanish]; Cefmetazolum [INN-Latin]; U-72791A; Cefmetazole (USP/INN); (6R,7S)-7-(2-((Cyanomethyl)thio)acetamido)-7-methoxy-3-(((1-methyl-1H-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid; (6R,7S)-7-(2-((Cyanomethyl)thio)acetamido)-7-methoxy-3-(((1-methyl-1H-tetrazol-5-yl)thiomethyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, sodium salt; (6R,7S)-7-({[(cyanomethyl)sulfanyl]acetyl}amino)-7-methoxy-3-{[(1-methyl-1H-tetrazol-5-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7S)-7-({[(cyanomethyl)thio]acetyl}amino)-7-(methyloxy)-3-{[(1-methyl-1H-tetrazol-5-yl)thio]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7S)-7-[[2-(cyanomethylsulfanyl)acetyl]amino]-7-methoxy-3-[(1-methyltetrazol-5-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; 6beta-{[(cyanomethyl)sulfanyl]acetamido}-6alpha-methoxy-3-{[(1-methyl-1H-tetrazol-5-yl)sulfanyl]methyl}ceph-3-em-4-carboxylic acid
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Bacterial infections [ICD9: 001-009, 010-018, 020-027, 030-041, 080-088, 090-099, 100-104; ICD10:A00-B99] | Approved | [538602] | ||
| Therapeutic Class |
Antibiotics
|
||||
| Company |
Pharmacia And Upjohn Co
|
||||
| Structure |
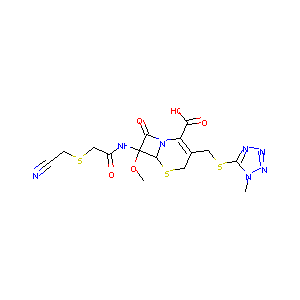
|
Download2D MOL |
|||
| Formula |
C15H17N7O5S3
|
||||
| Canonical SMILES |
CN1C(=NN=N1)SCC2=C(N3C(C(C3=O)(NC(=O)CSCC#N)OC)SC2)C(=O<br />)O
|
||||
| InChI |
1S/C15H17N7O5S3/c1-21-14(18-19-20-21)30-6-8-5-29-13-15(27-2,17-9(23)7-28-4-3-16)12(26)22(13)10(8)11(24)25/h13H,4-7H2,1-2H3,(H,17,23)(H,24,25)/t13-,15+/m1/s1
|
||||
| InChIKey |
SNBUBQHDYVFSQF-HIFRSBDPSA-N
|
||||
| CAS Number |
CAS 56796-20-4
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
10303, 632166, 7847973, 7978876, 8177398, 11466728, 11467848, 11486516, 15479323, 34707577, 46504461, 47217057, 47217058, 47440545, 47440546, 48259496, 48415716, 50050974, 50294685, 57312636, 58106756, 92251462, 92712300, 104338473, 123087792, 124766309, 126625684, 126686221, 131319170, 134224281, 134338424, 135002546, 135882605, 136357157, 137006381, 140396436, 152035638, 160963622, 162173144, 164788224, 175442148, 179150673, 184544918, 198970111, 223674319, 226513671, 251916490, 251917837, 252347145, 252449370
|
||||
| ChEBI ID |
ChEBI:3489
|
||||
| SuperDrug ATC ID |
J01DC09
|
||||
| SuperDrug CAS ID |
cas=056796204
|
||||
| Target and Pathway | |||||
| Target(s) | Penicillin binding protein 3 | Target Info | Binder | [538144] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

