Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D05LYX
|
||||
| Former ID |
DAP000037
|
||||
| Drug Name |
Glibenclamide
|
||||
| Synonyms |
Abbenclamide; Adiab; Azuglucon; Bastiverit; Benclamin; Betanase; Calabren; Cytagon; Daonil; Debtan; Diabeta; Diabiphage; Dibelet; Duraglucon; Euclamin; Euglucan; Euglucon; Euglykon; Gewaglucon; Gilemal; Glamide; Glibadone; Gliban; Gliben; Glibenbeta; Glibenclamida; Glibenclamidum; Glibenil; Glibens; Glibesyn; Glibet; Glibetic; Glibil; Gliboral; Glicem; Glidiabet; Glimel; Glimide; Glimidstata; Glisulin; Glitisol; Glubate; Gluben; Glucobene; Glucohexal; Glucolon; Glucomid; Glucoremed; Glucoven; Glyben; Glybenclamide; Glybenzcyclamide; Glyburide; Glycolande; Glycomin; Glynase; Hexaglucon; Humedia; Lederglib; Libanil; Lisaglucon; Maninil; Melix; Micronase; Miglucan; Nadib; Neogluconin; Normoglucon; Orabetic; Pira; Praeciglucon; PresTab; Prodiabet; Renabetic; Sugril; Suraben; Tiabet; Yuglucon; Euglucon N; Glibenclamid AL; Glibenclamid Basics; Glibenclamid Fabra; Glibenclamid Genericon; Glibenclamid Heumann; Glibenclamid Riker M; Glyburide [USAN]; Micronized glyburide; Betanese 5; Euglucon 5; G 0639; GBN 5; HB 419; HB 420; HB419; HB420; Norglicem 5; U 26452; UR 606; Apo-Glibenclamide; Daonil (TN); Dia-basan; Diabeta (TN); Euglucon (TN); Gen-Glybe; Gliben-Puren N; Glibenclamid Riker M.; Glibenclamid-Cophar; Glibenclamid-Ratiopharm; Glibenclamida [INN-Spanish]; Glibenclamidum [INN-Latin]; Gluco-Tablimen; Glyburide (USP); Glyburide (micronized); Glynase (TN); HB-419; HB-420; Hemi-Daonil; Med-Glionil; Micronase (TN); Novo-Glyburide; Semi-Euglucon; Semi-daonil; U-26452; Glibenclamide (JP15/INN); Semi-Daonil (TN); Semi-Gliben-Puren N; N-p-[2-(5-Chloro-2-methoxybenzamido)ethyl]benzenesulfonyl-N'-cyclohexylurea; N-p-[2-(5-Chloro-2-methoxybenzamido)-ethyl]benzene-sulfonyl-N-cyclohexylurea; N-(4-(2-(5-Chloro-2-methoxybenzamido)ethyl)phenylsulfonyl)-N'-cyclohexylurea; 1-((p-(2-(5-Chloro-o-anisamido)ethyl)phenyl)sulfonyl)-3-cyclohexylurea; 1-(p-(2-(5-Chloro-2-methoxybenzamido)ethyl)benzenesulfonyl)-3-cyclohexylurea; 5-Chloro-N-[4-(cyclohexylureidosulfonyl)phenethyl]-2-methoxybenzamide; 5-chloro-N-[2-[4-(cyclohexylcarbamoylsulfamoyl)phenyl]ethyl]-2-methoxybenzamide
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Hypoglycemic Agents
|
||||
| Company |
Nigerian-German Chemicals
|
||||
| Structure |
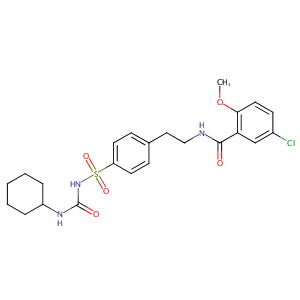
|
Download2D MOL |
|||
| Formula |
C23H28ClN3O5S
|
||||
| InChI |
InChI=1S/C23H28ClN3O5S/c1-32-21-12-9-17(24)15-20(21)22(28)25-14-13-16-7-10-19(11-8-16)33(30,31)27-23(29)26-18-5-3-2-4-6-18/h7-12,15,18H,2-6,13-14H2,1H3,(H,25,28)(H2,26,27,29)
|
||||
| InChIKey |
ZNNLBTZKUZBEKO-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 10238-21-8
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9234, 855894, 3146598, 5047958, 7847402, 7979403, 8147024, 8150168, 8152216, 10321542, 10502519, 11111219, 11112597, 11113353, 11119957, 11120445, 11120933, 11121416, 11121896, 11147040, 11335656, 11360895, 11362485, 11363144, 11365047, 11365706, 11367609, 11368268, 11370241, 11370242, 11372372, 11373210, 11375035, 11375771, 11376430, 11461867, 11466344, 11467464, 11485567, 11486267, 11489632, 11491142, 11493013, 11494064, 14835352, 17405062, 22391414, 24277838, 24895097, 26542333
|
||||
| ChEBI ID |
ChEBI:5441
|
||||
| SuperDrug ATC ID |
A10BB01
|
||||
| SuperDrug CAS ID |
cas=010238218
|
||||
| Target and Pathway | |||||
| Target(s) | Sulfonylurea receptor 1 | Target Info | Modulator | [556264] | |
| Pathway Interaction Database | FOXA2 and FOXA3 transcription factor networks | ||||
| PathWhiz Pathway | Muscle/Heart Contraction | ||||
| Pancreas Function | |||||
| WikiPathways | Potassium Channels | ||||
| Integration of energy metabolism | |||||
| References | |||||
| Ref 537022 | Emerging drug candidates of dipeptidyl peptidase IV (DPP IV) inhibitor class for the treatment of Type 2 Diabetes. Curr Drug Targets. 2009 Jan;10(1):71-87. | ||||
| Ref 539544 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2414). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

