Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D05LRD
|
||||
| Former ID |
DNCL001932
|
||||
| Drug Name |
BMS-933043
|
||||
| Indication | Psychiatric disorder [ICD9: 290-319; ICD10:F01-F99] | Phase 1 | [523918] | ||
| Company |
Bristol-Myers Squibb
|
||||
| Structure |
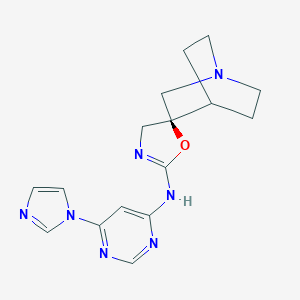
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Neuronal acetylcholine receptor protein, alpha-7 chain | Target Info | Modulator | [550057] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

