Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D04XIO
|
||||
| Former ID |
DIB013859
|
||||
| Drug Name |
Dutogliptin
|
||||
| Synonyms |
Dutogliptin tartrate; PHX-1004; PHX-1149; PHX-1149T; DPP IV inhibitors (diabetes), Phenomix; DPP IV program (diabetes), Phenomix
|
||||
| Indication | Type 2 diabetes [ICD9: 250; ICD10:E11] | Phase 3 | [1] | ||
| Company |
Phenomix Corp
|
||||
| Structure |
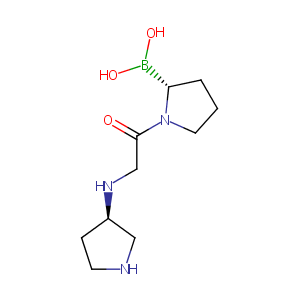
|
Download2D MOL |
|||
| Canonical SMILES |
N1C[C@@H](CC1)NCC(=O)N1[C@@H](CCC1)B(O)O
|
||||
| Target and Pathway | |||||
| Target(s) | Dipeptidyl peptidase IV | Target Info | Inhibitor | [2] | |
| KEGG Pathway | Protein digestion and absorption | ||||
| NetPath Pathway | IL2 Signaling Pathway | ||||
| TGF_beta_Receptor Signaling Pathway | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT00690638) Safety and Efficacy Study of Dutogliptin/PHX1149T to Treat Type 2 Diabetes Mellitus. U.S. National Institutes of Health. | ||||
| REF 2 | Dutogliptin, a selective DPP4 inhibitor, improves glycaemic control in patients with type 2 diabetes: a 12-week, double-blind, randomized, placebo-controlled, multicentre trial. Diabetes Obes Metab. 2010 Apr;12(4):348-55. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

