Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D04WPG
|
||||
| Former ID |
DAP000831
|
||||
| Drug Name |
Valproic Acid
|
||||
| Synonyms |
Avugane; Baceca; Convulex; Convulsofin; Depakene; Depakin; Depakine; Deproic; Dipropylacetate; Ergenyl; Mylproin; Savicol; Stavzor; VPA; Valproate; Valproinsaeure; Vupral; Acide valproique; Acido valproico; Acidum valproicum; Depakin chrono; Depakine chrono; Dipropyl Acetate; Dipropylacetic acid; Med Valproic; Myproic Acid; Propylvaleric acid; Semisodium Valproate; Valproic acid USP; Abbott 44090; Valproic acid USP24; Acide valproique [INN-French]; Acido valproico [INN-Spanish]; Acidum valproicum [INN-Latin]; Alti-Valproic; Convulex (TN); Depacon (TN); Depakene (TN); Depakote (TM); Depakote (TN); Depakote ER (TN); Dom-Valproic; Encorate (TN); Epilim (TN); Epival (TN); G2M-777; Kyselina 2-propylvalerova; Kyselina 2-propylvalerova [Czech]; N-DPA; N-Dipropylacetic acid; Novo-Valproic; Nu-Valproic; PMS-Valproic Acid; Penta-Valproic; Stavzor (TN); Valproic acid (USP); Winthrop (TN); Di-n-propylacetic acid; Di-n-propylessigsaeure; Di-n-propylessigsaure; Di-n-propylessigsaure [German]; Valproic acid [USAN:INN:BAN]; Valproic Acid, Sodium Salt (2:1); 2 PP (base); 2-Propylpentanoic acid; 2-Propylvaleric acid; 2-n-Propyl-n-valeric acid; 4-Heptanecarboxylic acid
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Anticonvulsants
|
||||
| Company |
Abbott Laboratories; TopoTarget
|
||||
| Structure |
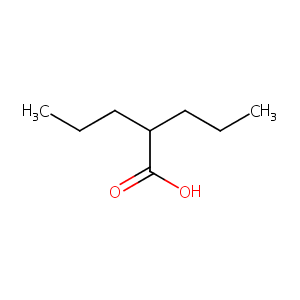
|
Download2D MOL |
|||
| Formula |
C8H16O2
|
||||
| InChI |
InChI=1S/C8H16O2/c1-3-5-7(6-4-2)8(9)10/h7H,3-6H2,1-2H3,(H,9,10)
|
||||
| InChIKey |
NIJJYAXOARWZEE-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 99-66-1
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9394, 399407, 621684, 3138784, 4918409, 7847465, 7980632, 8151988, 10539166, 11335448, 11360687, 11363415, 11364691, 11365977, 11367253, 11368539, 11369815, 11372725, 11372856, 11374010, 11375415, 11376701, 11377978, 11461659, 11484648, 11488762, 11491548, 11492191, 11494335, 12015354, 14710660, 15321539, 17389523, 24898751, 26697333, 26752920, 26752921, 29222263, 46260925, 46505925, 48416692, 48424277, 48425730, 49635685, 49640649, 49856166, 50062089, 50105536, 53788878, 56310655
|
||||
| ChEBI ID |
ChEBI:39867
|
||||
| SuperDrug ATC ID |
N03AG01
|
||||
| SuperDrug CAS ID |
cas=000099661
|
||||
| Target and Pathway | |||||
| References | |||||
| Ref 536447 | Emerging disease-modifying therapies for the treatment of motor neuron disease/amyotropic lateral sclerosis. Expert Opin Emerg Drugs. 2007 May;12(2):229-52. | ||||
| Ref 542039 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7009). | ||||
| Ref 551871 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
| Ref 535221 | The new generation of GABA enhancers. Potential in the treatment of epilepsy. CNS Drugs. 2001;15(5):339-50. | ||||
| Ref 536272 | Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006 Sep;5(9):769-84. | ||||
| Ref 537490 | Histone deacetylase inhibitors induce apoptosis, histone hyperacetylation and up-regulation of gene transcription in Schistosoma mansoni. Mol Biochem Parasitol. 2009 Jun 16. | ||||
| Ref 537523 | Histone Deacetylase 2 is a Key Regulator of Diabetes and Transforming Growth Factor-{beta}1-Induced Renal Injury. Am J Physiol Renal Physiol. 2009 Jun 24. | ||||
| Ref 537552 | Transcription-independent heritability of induced histone modifications in the mouse preimplantation embryo. PLoS One. 2009 Jun 30;4(6):e6086. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

