Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D02ZHW
|
||||
| Former ID |
DIB016950
|
||||
| Drug Name |
177Lu-DOTA-octreotate (neuroendocrine tumors), Advanced Accelerator Applications
|
||||
| Synonyms |
Lutate; Lutathera; Octreotate [177Lu]; Lutetium-177 octreotate; 177-Lu octreotate; 177-Lu-radiolabeled somatostatin receptor-targeted peptide (neuroendocrine tumor), Biosynthema; 177-lutetium-radiolabeled somatostatin receptor-targeted peptide (neuroendocrinetumor), Biosynthema; 177Lutetium DOTA0-Tyr3-Octreotate
|
||||
| Indication | Melanoma [ICD9: 172; ICD10:C43] | Phase 3 | [523870] | ||
| Company |
Advanced accelerator applications
|
||||
| Structure |
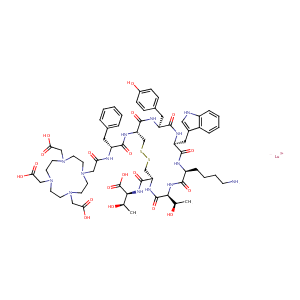
|
Download2D MOL |
|||
| Canonical SMILES |
N([C@H](C(=O)O)[C@H](O)C)C(=O)[C@@H]1CSSC[C@H](NC(=O)[C<br />@H](NC(=O)CN2CCN(CC(=O)O)CCN(CC(=O)O)CCN(CC(=O)O)CC2)Cc<br />2ccccc2)C(=O)N[C@H](C(=O)N[C@H](C(=O)N[C@H](C(=O)N[C@H]<br />(C(=O)N1)[C@H](O)C)CCCCN)Cc1c[nH]c2c1cccc2)Cc1ccc(cc1)O<br />.[177Lu+3]
|
||||
| Target and Pathway | |||||
| Target(s) | Somatostatin receptor | Target Info | Modulator | [532708] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

