Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D02ZBW
|
||||
| Former ID |
DIB005411
|
||||
| Drug Name |
LC-150444
|
||||
| Synonyms |
LCD15-0444; Dipeptidyl peptidase IV inhibitors (oral, type 2 diabetes), LG Life Sciences
|
||||
| Indication | Type 2 diabetes [ICD9: 250; ICD10:E11] | Phase 3 | [1] | ||
| Company |
LG Life Sciences Ltd
|
||||
| Structure |
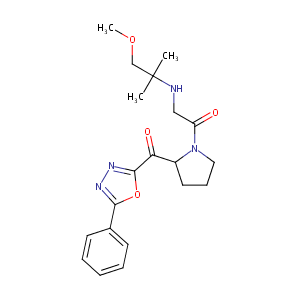
|
Download2D MOL |
|||
| Canonical SMILES |
N1(CCCC1C(=O)c1oc(nn1)c1ccccc1)C(=O)CNC(COC)(C)C
|
||||
| Target and Pathway | |||||
| Target(s) | Dipeptidyl peptidase IV | Target Info | Inhibitor | [2] | |
| KEGG Pathway | Protein digestion and absorption | ||||
| NetPath Pathway | IL2 Signaling Pathway | ||||
| TGF_beta_Receptor Signaling Pathway | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT02089126) Phase III Trial to Evaluate the Efficacy and Safety of Gemigliptin 50mg qd Added to Ongoing Glimepiride as Fix-dose Combination in Patients With Type 2 Diabetes. U.S.National Institutes of Health. | ||||
| REF 2 | Clinical pipeline report, company report or official report of ShangHai APIs Chemical. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

