Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01ZLU
|
||||
| Former ID |
DIB010879
|
||||
| Drug Name |
TAS-120
|
||||
| Indication | Multiple myeloma [ICD9: 203; ICD10:C90] | Phase 1/2 | [1] | ||
| Company |
Taiho oncology
|
||||
| Structure |
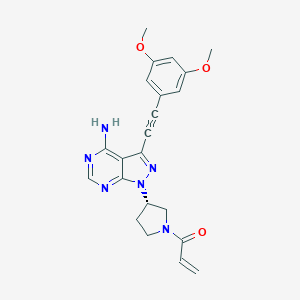
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Fibroblast growth factor receptor | Target Info | Antagonist | [2] | |
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT02052778) A Dose Finding Study Followed by a Safety and Efficacy Study in Patients With Advanced Solid Tumors or Multiple Myeloma With FGF/FGFR-Related Abnormalities. U.S. National Institutes of Health. | ||||
| REF 2 | TAS-120, a highly potent and selective irreversible FGFR inhibitor, is effective in tumors harboring various FGFR gene abnormalities. Molecular Cancer Therapeutics. 01/2014; 12(11_Supplement):A270-A270. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

