Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01TCB
|
||||
| Former ID |
DNCL001974
|
||||
| Drug Name |
ELND005
|
||||
| Indication | Mild to moderate alzheimer disease [ICD9: 331; ICD10:G30] | Phase 2 | [1] | ||
| Company |
Elan; Transition Therapeutics
|
||||
| Structure |
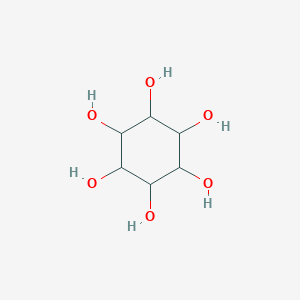
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Amyloid beta A4 protein | Target Info | Modulator | [2] | |
| KEGG Pathway | Notch signaling pathway | ||||
| Alzheimer's disease | |||||
| Reactome | Nuclear signaling by ERBB4 | ||||
| Degradation of the extracellular matrix | |||||
| Regulated proteolysis of p75NTR | |||||
| NRIF signals cell death from the nucleus | |||||
| Activated NOTCH1 Transmits Signal to the Nucleus | |||||
| Constitutive Signaling by NOTCH1 PEST Domain Mutants | |||||
| Constitutive Signaling by NOTCH1 HD+PEST Domain Mutants | |||||
| EPH-ephrin mediated repulsion of cells | |||||
| WikiPathways | Notch Signaling Pathway | ||||
| Signaling by ERBB4 | |||||
| Signaling by NOTCH3 | |||||
| Signaling by NOTCH4 | |||||
| Signaling by NOTCH1 | |||||
| Signaling by NOTCH2 | |||||
| Notch Signaling Pathway | |||||
| Alzheimers Disease | |||||
| Signalling by NGF | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT01766336) A 36-Week Safety Extension Study of ELND005 as a Treatment for Agitation and Aggression in Alzheimer's Disease. U.S. National Institutes of Health. | ||||
| REF 2 | A phase 2 randomized trial of ELND005, scyllo-inositol, in mild to moderate Alzheimer disease. Neurology. 2011 Sep 27;77(13):1253-62. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

