Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01SZA
|
||||
| Former ID |
DIB016738
|
||||
| Drug Name |
MR-1817
|
||||
| Indication | Pain [ICD9: 338, 356.0, 356.8,780; ICD10:G64, G90.0, R52, G89] | Phase 1 | [522759] | ||
| Company |
Mochida Pharmaceutical Co Ltd
|
||||
| Structure |
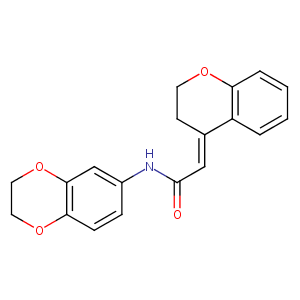
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Vanilloid receptor 1 | Target Info | Antagonist | [548951] | |
| NetPath Pathway | IL2 Signaling Pathway | ||||
| Pathway Interaction Database | Trk receptor signaling mediated by the MAPK pathway | ||||
| Trk receptor signaling mediated by PI3K and PLC-gamma | |||||
| Reactome | TRP channels | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

