Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D00BRD
|
||||
| Former ID |
DNC006144
|
||||
| Drug Name |
4-hydroxybenzaldehyde
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [1] | ||
| Structure |
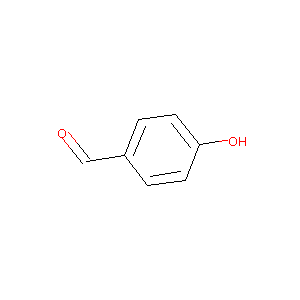
|
Download2D MOL |
|||
| Formula |
C7H6O2
|
||||
| Canonical SMILES |
C1=CC(=CC=C1C=O)O
|
||||
| InChI |
1S/C7H6O2/c8-5-6-1-3-7(9)4-2-6/h1-5,9H
|
||||
| InChIKey |
RGHHSNMVTDWUBI-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | 4-aminobutyrate aminotransferase, mitochondrial | Target Info | Inhibitor | [1] | |
| BioCyc Pathway | GABA shunt | ||||
| Valine degradation | |||||
| Beta-alanine degradation | |||||
| 4-aminobutyrate degradation | |||||
| KEGG Pathway | Alanine, aspartate and glutamate metabolism | ||||
| Valine, leucine and isoleucine degradation | |||||
| beta-Alanine metabolism | |||||
| Propanoate metabolism | |||||
| Butanoate metabolism | |||||
| Metabolic pathways | |||||
| GABAergic synapse | |||||
| PANTHER Pathway | Aminobutyrate degradation | ||||
| Pyrimidine Metabolism | |||||
| Gamma-aminobutyric acid synthesis | |||||
| PathWhiz Pathway | Aspartate Metabolism | ||||
| Glutamate Metabolism | |||||
| Beta-Alanine Metabolism | |||||
| Valine, Leucine and Isoleucine Degradation | |||||
| Propanoate Metabolism | |||||
| WikiPathways | GABA synthesis, release, reuptake and degradation | ||||
| Alanine and aspartate metabolism | |||||
| References | |||||
| REF 1 | Bioorg Med Chem Lett. 2009 Feb 1;19(3):731-4. Epub 2008 Dec 11.Inactivation of GABA transaminase by 3-chloro-1-(4-hydroxyphenyl)propan-1-one. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

