Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T97621
|
|||||
| Target Name |
cAMP-dependent chloride channel F508 deletion (CFTR del F508)
|
|||||
| Synonyms |
cAMP-dependent chloride channel (del F508); Cystic fibrosis transmembrane conductance regulator (del F508); Channel conductance-controlling ATPase; CFTR; ATP-binding cassette sub-family C member 7 (del F508); ABCC7 (del F508)
Click to Show/Hide
|
|||||
| Gene Name |
CFTR
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Cystic fibrosis [ICD-11: CA25] | |||||
| 2 | Intrathoracic organs injury [ICD-11: NB32] | |||||
| Function |
Epithelial ion channel that plays an important role in the regulation of epithelial ion and water transport and fluid homeostasis. Mediates the transport of chloride ions across the cell membrane. Channel activity is coupled to ATP hydrolysis. The ion channel is also permeable to HCO(3-); selectivity depends on the extracellular chloride concentration. Exerts its function also by modulating the activity of other ion channels and transporters. Plays an important role in airway fluid homeostasis. Contributes to the regulation of the pH and the ion content of the airway surface fluid layer and thereby plays an important role in defense against pathogens. Modulates the activity of the epithelial sodium channel (ENaC) complex, in part by regulating the cell surface expression of the ENaC complex. Inhibits the activity of the ENaC channel containing subunits SCNN1A, SCNN1B and SCNN1G. Inhibits the activity of the ENaC channel containing subunits SCNN1D, SCNN1B and SCNN1G, but not of the ENaC channel containing subunits SCNN1A, SCNN1B and SCNN1G. May regulate bicarbonate secretion and salvage in epithelial cells by regulating the transporter SLC4A7. Can inhibit the chloride channel activity of ANO1. Plays a role in the chloride and bicarbonate homeostasis during sperm epididymal maturation and capacitation.
Click to Show/Hide
|
|||||
| BioChemical Class |
ABC transporter
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 5.6.1.6
|
|||||
| Sequence |
MQRSPLEKASVVSKLFFSWTRPILRKGYRQRLELSDIYQIPSVDSADNLSEKLEREWDRE
LASKKNPKLINALRRCFFWRFMFYGIFLYLGEVTKAVQPLLLGRIIASYDPDNKEERSIA IYLGIGLCLLFIVRTLLLHPAIFGLHHIGMQMRIAMFSLIYKKTLKLSSRVLDKISIGQL VSLLSNNLNKFDEGLALAHFVWIAPLQVALLMGLIWELLQASAFCGLGFLIVLALFQAGL GRMMMKYRDQRAGKISERLVITSEMIENIQSVKAYCWEEAMEKMIENLRQTELKLTRKAA YVRYFNSSAFFFSGFFVVFLSVLPYALIKGIILRKIFTTISFCIVLRMAVTRQFPWAVQT WYDSLGAINKIQDFLQKQEYKTLEYNLTTTEVVMENVTAFWEEGFGELFEKAKQNNNNRK TSNGDDSLFFSNFSLLGTPVLKDINFKIERGQLLAVAGSTGAGKTSLLMVIMGELEPSEG KIKHSGRISFCSQFSWIMPGTIKENIIFGVSYDEYRYRSVIKACQLEEDISKFAEKDNIV LGEGGITLSGGQRARISLARAVYKDADLYLLDSPFGYLDVLTEKEIFESCVCKLMANKTR ILVTSKMEHLKKADKILILHEGSSYFYGTFSELQNLQPDFSSKLMGCDSFDQFSAERRNS ILTETLHRFSLEGDAPVSWTETKKQSFKQTGEFGEKRKNSILNPINSIRKFSIVQKTPLQ MNGIEEDSDEPLERRLSLVPDSEQGEAILPRISVISTGPTLQARRRQSVLNLMTHSVNQG QNIHRKTTASTRKVSLAPQANLTELDIYSRRLSQETGLEISEEINEEDLKECFFDDMESI PAVTTWNTYLRYITVHKSLIFVLIWCLVIFLAEVAASLVVLWLLGNTPLQDKGNSTHSRN NSYAVIITSTSSYYVFYIYVGVADTLLAMGFFRGLPLVHTLITVSKILHHKMLHSVLQAP MSTLNTLKAGGILNRFSKDIAILDDLLPLTIFDFIQLLLIVIGAIAVVAVLQPYIFVATV PVIVAFIMLRAYFLQTSQQLKQLESEGRSPIFTHLVTSLKGLWTLRAFGRQPYFETLFHK ALNLHTANWFLYLSTLRWFQMRIEMIFVIFFIAVTFISILTTGEGEGRVGIILTLAMNIM STLQWAVNSSIDVDSLMRSVSRVFKFIDMPTEGKPTKSTKPYKNGQLSKVMIIENSHVKK DDIWPSGGQMTVKDLTAKYTEGGNAILENISFSISPGQRVGLLGRTGSGKSTLLSAFLRL LNTEGEIQIDGVSWDSITLQQWRKAFGVIPQKVFIFSGTFRKNLDPYEQWSDQEIWKVAD EVGLRSVIEQFPGKLDFVLVDGGCVLSHGHKQLMCLARSVLSKAKILLLDEPSAHLDPVT YQIIRRTLKQAFADCTVILCEHRIEAMLECQQFLVIEENKVRQYDSIQKLLNERSLFRQA ISPSDRVKLFPHRNSSKCKSKPQIAALKEETEEEVQDTRL Click to Show/Hide
|
|||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 1 Approved Drugs | + | ||||
| 1 | Lumacaftor + ivacaftor | Drug Info | Approved | Cystic fibrosis | [1] | |
| Mode of Action | [+] 1 Modes of Action | + | ||||
| Enhancer | [+] 1 Enhancer drugs | + | ||||
| 1 | Lumacaftor + ivacaftor | Drug Info | [1] | |||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Ivacaftor | Ligand Info | |||||
| Structure Description | The complex of phosphorylated human delta F508 cystic fibrosis transmembrane conductance regulator (CFTR) with Trikafta [elexacaftor (VX-445), tezacaftor (VX-661), ivacaftor (VX-770)] and ATP/Mg | PDB:8EIQ | ||||
| Method | Electron microscopy | Resolution | 3.00 Å | Mutation | Yes | [2] |
| PDB Sequence |
MQRSPLEKAS
10 VVSKLFFSWT20 RPILRKGYRQ30 RLELSDIYQI40 PSVDSADNLS50 EKLEREWDRE 60 LASKKNPKLI70 NALRRCFFWR80 FMFYGIFLYL90 GEVTKAVQPL100 LLGRIIASYD 110 PDNKEERSIA120 IYLGIGLCLL130 FIVRTLLLHP140 AIFGLHHIGM150 QMRIAMFSLI 160 YKKTLKLSSR170 VLDKISIGQL180 VSLLSNNLNK190 FDEGLALAHF200 VWIAPLQVAL 210 LMGLIWELLQ220 ASAFCGLGFL230 IVLALFQAGL240 GRMMMKYRDQ250 RAGKISERLV 260 ITSEMIENIQ270 SVKAYCWEEA280 MEKMIENLRQ290 TELKLTRKAA300 YVRYFNSSAF 310 FFSGFFVVFL320 SVLPYALIKG330 IILRKIFTTI340 SFCIVLRMAV350 TRQFPWAVQT 360 WYDSLGAINK370 IQDFLQKQEY380 KTLEYNLTTT390 EVVMENVTAF400 WEGTPVLKDI 444 NFKIERGQLL454 AVAGSTGAGK464 TSLLMVIMGE474 LEPSEGKIKH484 SGRISFCSQF 494 SWIMPGTIKE504 NIIGVSYDEY515 RYRSVIKACQ525 LEEDISKFAE535 KDNIVLGEGG 545 ITLSGGQRAR555 ISLARAVYKD565 ADLYLLDSPF575 GYLDVLTEKE585 IFESCVCKLM 595 ANKTRILVTS605 KMEHLKKADK615 ILILHEGSSY625 FYGTFSELQN635 LWNTYLRYIT 854 VHKSLIFVLI864 WCLVIFLAEV874 AASLVVLWLL884 GSYAVIITST910 SSYYVFYIYV 920 GVADTLLAMG930 FFRGLPLVHT940 LITVSKILHH950 KMLHSVLQAP960 MSTLNTLKAG 970 GILNRFSKDI980 AILDDLLPLT990 IFDFIQLLLI1000 VIGAIAVVAV1010 LQPYIFVATV 1020 PVIVAFIMLR1030 AYFLQTSQQL1040 KQLESEGRSP1050 IFTHLVTSLK1060 GLWTLRAFGR 1070 QPYFETLFHK1080 ALNLHTANWF1090 LYLSTLRWFQ1100 MRIEMIFVIF1110 FIAVTFISIL 1120 TTGEGEGRVG1130 IILTLAMNIM1140 STLQWAVNSS1150 IDVDSLMRSV1160 SRVFKFIDMP 1170 TEGIWPSGGQ1209 MTVKDLTAKY1219 TEGGNAILEN1229 ISFSISPGQR1239 VGLLGRTGSG 1249 KSTLLSAFLR1259 LLNTEGEIQI1269 DGVSWDSITL1279 QQWRKAFGVI1289 PQKVFIFSGT 1299 FRKNLDPYEQ1309 WSDQEIWKVA1319 DEVGLRSVIE1329 QFPGKLDFVL1339 VDGGCVLSHG 1349 HKQLMCLARS1359 VLSKAKILLL1369 DQPSAHLDPV1379 TYQIIRRTLK1389 QAFADCTVIL 1399 CEHRIEAMLE1409 CQQFLVIEEN1419 KVRQYDSIQK1429 LLNERSLFRQ1439 AISPSDRVKL 1449 FP
|
|||||
|
|
||||||
| Ligand Name: Adenosine triphosphate | Ligand Info | |||||
| Structure Description | Human CFTR first nucleotide binding domain with dF508/V510D | PDB:6WBS | ||||
| Method | X-ray diffraction | Resolution | 1.86 Å | Mutation | Yes | [3] |
| PDB Sequence |
TTTEVVMENV
429 TAFWEEGGTP439 VLKDINFKIE449 RGQLLAVAGS459 TGAGKTSLLM469 VIMGELEPSE 479 GKIKHSGRIS489 FCSQFSWIMP499 GTIKENIIGD510 SYDEYRYRSV520 IKACQLEEDI 530 SKFAEKDNIV540 LGEGGITLSG550 GQRARISLAR560 AVYKDADLYL570 LDSPFGYLDV 580 LTEKEIFESC590 VCKLMANKTR600 ILVTSKMEHL610 KKADKILILH620 EGSSYFYGTF 630 SELQN
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Human Pathway Affiliation
|
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| ABC transporters | hsa02010 | Affiliated Target |
|
| Class: Environmental Information Processing => Membrane transport | Pathway Hierarchy | ||
| cAMP signaling pathway | hsa04024 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| AMPK signaling pathway | hsa04152 | Affiliated Target |
|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Tight junction | hsa04530 | Affiliated Target |
|
| Class: Cellular Processes => Cellular community - eukaryotes | Pathway Hierarchy | ||
| Gastric acid secretion | hsa04971 | Affiliated Target |
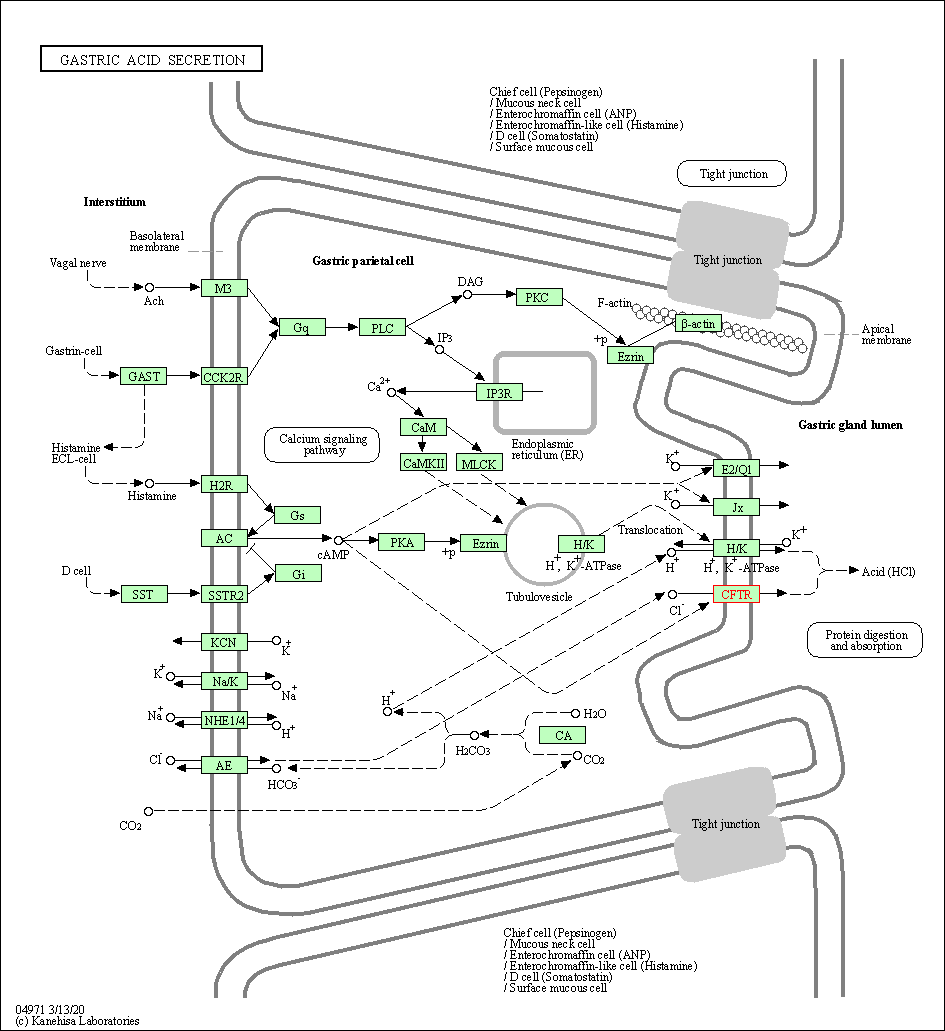
|
| Class: Organismal Systems => Digestive system | Pathway Hierarchy | ||
| Pancreatic secretion | hsa04972 | Affiliated Target |
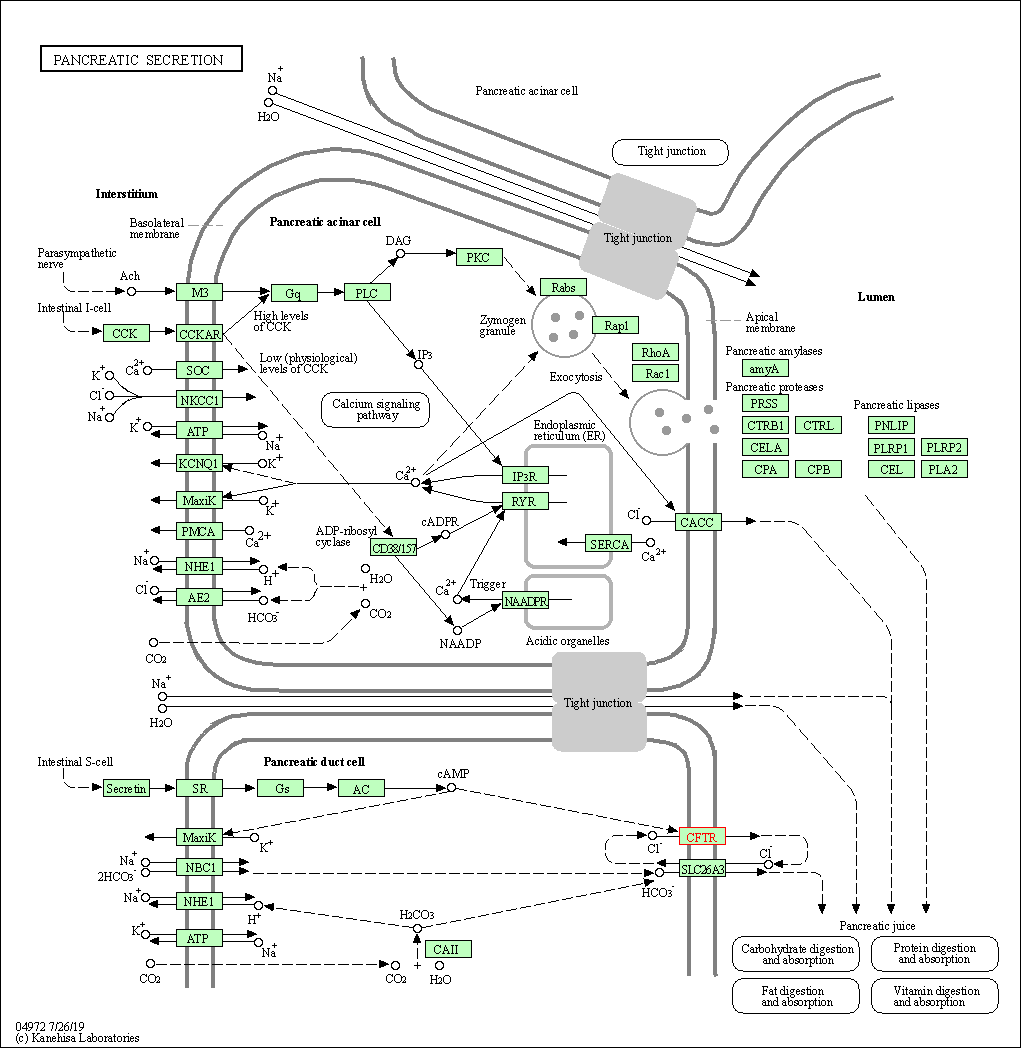
|
| Class: Organismal Systems => Digestive system | Pathway Hierarchy | ||
| Bile secretion | hsa04976 | Affiliated Target |
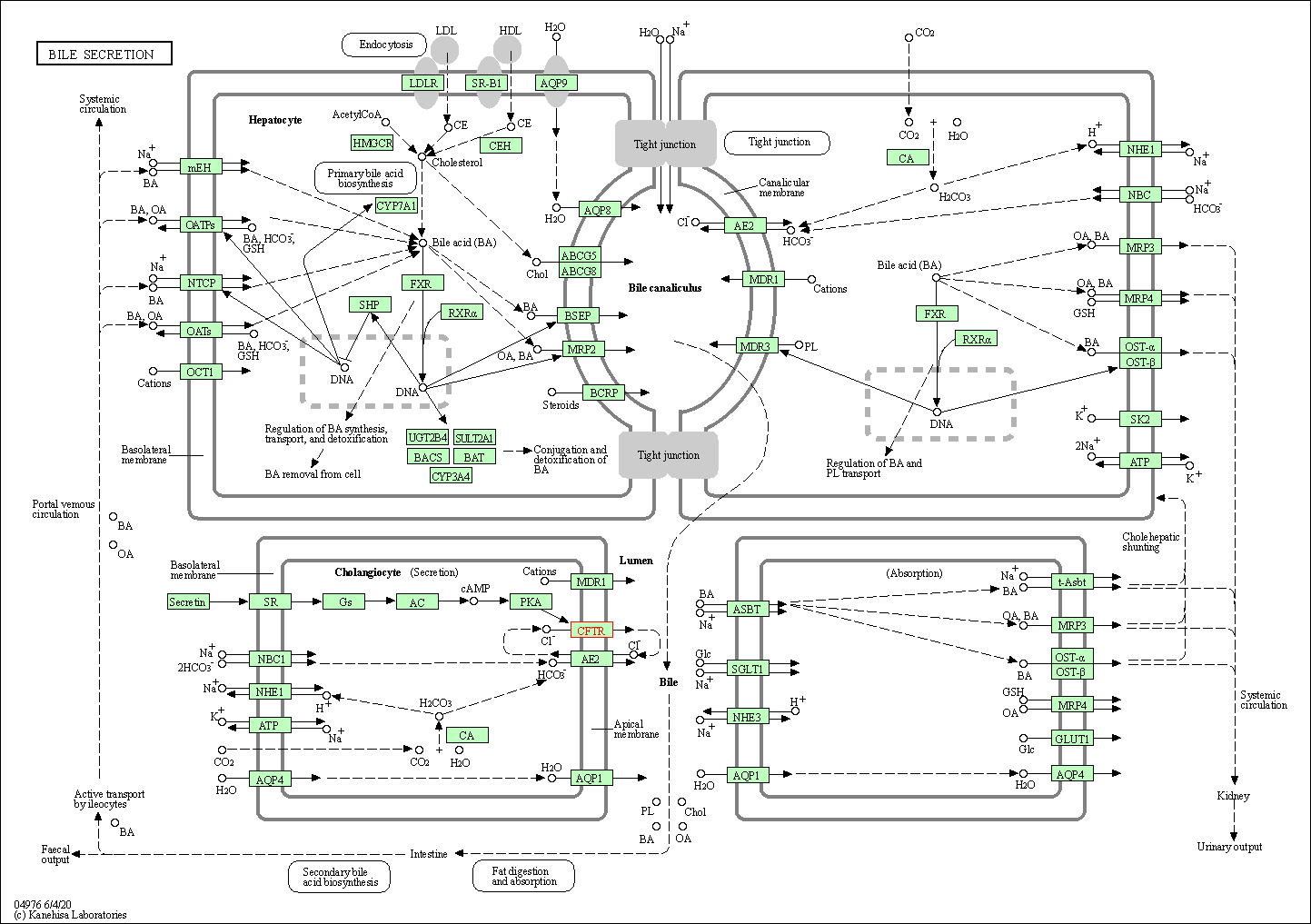
|
| Class: Organismal Systems => Digestive system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Antibodies and venom peptides: new modalities for ion channels. Nat Rev Drug Discov. 2019 May;18(5):339-357. | |||||
| REF 2 | Molecular structures reveal synergistic rescue of Delta508 CFTR by Trikafta modulators. Science. 2022 Oct 21;378(6617):284-290. | |||||
| REF 3 | Determining the Molecular Mechanism of Suppressor Mutation V510D and the Contribution of Helical Unraveling to the dF508-CFTR Defect | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

