Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T94479
(Former ID: TTDC00145)
|
|||||
| Target Name |
Ribosomal protein S6 kinase beta-1 (S6K1)
|
|||||
| Synonyms |
p70-S6K 1; p70 ribosomal S6 kinase alpha; p70 S6KA; p70 S6K-alpha; p70 S6 kinase alpha; Serine/threonine-protein kinase 14A; STK14A; S6K-beta-1; S6K; Ribosomal protein S6 kinase I; P70S6K1; P70-S6K; 70 kDa ribosomal protein S6 kinase 1
Click to Show/Hide
|
|||||
| Gene Name |
RPS6KB1
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 3 Target-related Diseases | + | ||||
| 1 | Alzheimer disease [ICD-11: 8A20] | |||||
| 2 | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||||
| 3 | Ulcerative colitis [ICD-11: DD71] | |||||
| Function |
Regulates protein synthesis through phosphorylation of EIF4B, RPS6 and EEF2K, and contributes to cell survival by repressing the pro-apoptotic function of BAD. Under conditions of nutrient depletion, the inactive form associates with the EIF3 translation initiation complex. Upon mitogenic stimulation, phosphorylation by the mammalian target of rapamycin complex 1 (mTORC1) leads to dissociation from the EIF3 complex and activation. The active form then phosphorylates and activates several substrates in the pre-initiation complex, including the EIF2B complex and the cap-binding complex component EIF4B. Also controls translation initiation by phosphorylating a negative regulator of EIF4A, PDCD4, targeting it for ubiquitination and subsequent proteolysis. Promotes initiation of the pioneer round of protein synthesis by phosphorylating POLDIP3/SKAR. In response to IGF1, activates translation elongation by phosphorylating EEF2 kinase (EEF2K), which leads to its inhibition and thus activation of EEF2. Also plays a role in feedback regulation of mTORC2 by mTORC1 by phosphorylating RICTOR, resulting in the inhibition of mTORC2 and AKT1 signaling. Mediates cell survival by phosphorylating the pro-apoptotic protein BAD and suppressing its pro-apoptotic function. Phosphorylates mitochondrial URI1 leading to dissociation of a URI1-PPP1CC complex. The free mitochondrial PPP1CC can then dephosphorylate RPS6KB1 at Thr-412, which is proposed to be a negative feedback mechanism for the RPS6KB1 anti-apoptotic function. Mediates TNF-alpha-induced insulin resistance by phosphorylating IRS1 at multiple serine residues, resulting in accelerated degradation of IRS1. In cells lacking functional TSC1-2 complex, constitutively phosphorylates and inhibits GSK3B. May be involved in cytoskeletal rearrangement through binding to neurabin. Phosphorylates and activates the pyrimidine biosynthesis enzyme CAD, downstream of MTOR. Following activation by mTORC1, phosphorylates EPRS and thereby plays a key role in fatty acid uptake by adipocytes and also most probably in interferon-gamma-induced translation inhibition. Serine/threonine-protein kinase that acts downstream of mTOR signaling in response to growth factors and nutrients to promote cell proliferation, cell growth and cell cycle progression.
Click to Show/Hide
|
|||||
| BioChemical Class |
Kinase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 2.7.11.1
|
|||||
| Sequence |
MRRRRRRDGFYPAPDFRDREAEDMAGVFDIDLDQPEDAGSEDELEEGGQLNESMDHGGVG
PYELGMEHCEKFEISETSVNRGPEKIRPECFELLRVLGKGGYGKVFQVRKVTGANTGKIF AMKVLKKAMIVRNAKDTAHTKAERNILEEVKHPFIVDLIYAFQTGGKLYLILEYLSGGEL FMQLEREGIFMEDTACFYLAEISMALGHLHQKGIIYRDLKPENIMLNHQGHVKLTDFGLC KESIHDGTVTHTFCGTIEYMAPEILMRSGHNRAVDWWSLGALMYDMLTGAPPFTGENRKK TIDKILKCKLNLPPYLTQEARDLLKKLLKRNAASRLGAGPGDAGEVQAHPFFRHINWEEL LARKVEPPFKPLLQSEEDVSQFDSKFTRQTPVDSPDDSTLSESANQVFLGFTYVAPSVLE SVKEKFSFEPKIRSPRRFIGSPRTPVSPVKFSPGDFWGRGASASTANPQTPVEYPMETSG IEQMDVTMSGEASAPLPIRQPNSGPYKKQAFPMISKRPEHLRMNL Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| ADReCS ID | BADD_A05675 | |||||
| HIT2.0 ID | T40NB1 | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 3 Clinical Trial Drugs | + | ||||
| 1 | M2698 | Drug Info | Phase 1 | Solid tumour/cancer | [2] | |
| 2 | NNI-362 | Drug Info | Phase 1 | Alzheimer disease | [3] | |
| 3 | PF-4708671 | Drug Info | Clinical trial | Ulcerative colitis | [4] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Inhibitor | [+] 16 Inhibitor drugs | + | ||||
| 1 | M2698 | Drug Info | [5] | |||
| 2 | PF-4708671 | Drug Info | [4] | |||
| 3 | PMID27410995-Compound-Figure3c | Drug Info | [7] | |||
| 4 | Pyrimido[4,5-d] pyrimidines and pyrido[4,3-d] pyrimidine derivative 1 | Drug Info | [7] | |||
| 5 | 4,5,6,7-tetrabromo-1H-benzo[d][1,2,3]triazole | Drug Info | [8] | |||
| 6 | 4-[(3,5-diamino-1H-pyrazol-4-yl)diazenyl]phenol | Drug Info | [9] | |||
| 7 | ACTB-1003 | Drug Info | [10] | |||
| 8 | CI-1040 | Drug Info | [1] | |||
| 9 | KN-62 | Drug Info | [1] | |||
| 10 | KT-5720 | Drug Info | [1] | |||
| 11 | PMID20005102C1 | Drug Info | [11] | |||
| 12 | RO-316233 | Drug Info | [1] | |||
| 13 | Ro31-8220 | Drug Info | [1] | |||
| 14 | SB-415286 | Drug Info | [12] | |||
| 15 | SB-747651A | Drug Info | [13] | |||
| 16 | STAUROSPORINONE | Drug Info | [1] | |||
| Stimulator | [+] 1 Stimulator drugs | + | ||||
| 1 | NNI-362 | Drug Info | [6] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: M2698 | Ligand Info | |||||
| Structure Description | P70 S6K1 IN COMPLEX WITH MSC2363318A-1 | PDB:7N93 | ||||
| Method | X-ray diffraction | Resolution | 2.74 Å | Mutation | Yes | [14] |
| PDB Sequence |
RPECFELLRV
96 LGKGGYGKVF106 QVRKVTGANT116 GKIFAMKVLK126 KAMIVRNAKD136 TAHTKAERNI 146 LEEVKHPFIV156 DLIYAFQTGG166 KLYLILEYLS176 GGELFMQLER186 EGIFMEDTAC 196 FYLAEISMAL206 GHLHQKGIIY216 RDLKPENIML226 NHQGHVKLTD236 FGLCKECGTI 257 EYMAPEILMR267 SGHNRAVDWW277 SLGALMYDML287 TGAPPFTGEN297 RKKTIDKILK 307 CKLNLPPYLT317 QEARDLLKKL327 LKRNAASRLG337 AGPGDAGEVQ347 AHPFFRHINW 357 EELLARKVEP367 PFKPLLQSEE377 DVSQFDSKFT387 RQPVDPNQVF408 LGFEYVAPSV 418
|
|||||
|
|
LEU97
3.994
GLY98
3.221
LYS99
3.271
GLY100
3.275
TYR102
4.294
GLY103
3.532
LYS104
3.278
VAL105
3.188
ALA121
3.176
LYS123
3.276
VAL124
4.817
LEU125
3.623
VAL156
4.153
LEU172
3.431
|
|||||
| Ligand Name: PF-4708671 | Ligand Info | |||||
| Structure Description | Crystal structure of S6K1 kinase domain in complex with a pyrimidine derivative PF-4708671 2-{[4-(5-ethylpyrimidin-4-yl)piperazin-1-yl]methyl}-5-(trifluoromethyl)-1H-benzimidazole | PDB:3WE4 | ||||
| Method | X-ray diffraction | Resolution | 2.00 Å | Mutation | No | [15] |
| PDB Sequence |
KIRPECFELL
94 RVLGKGGYGK104 VFQVRKVTGA114 NTGKIFAMKV124 LKKAMIVRNA134 KDTAHTKAER 144 NILEEVKHPF154 IVDLIYAFQT164 GGKLYLILEY174 LSGGELFMQL184 EREGIFMEDT 194 ACFYLAEISM204 ALGHLHQKGI214 IYRDLKPENI224 MLNHQGHVKL234 TDFGLCKESI 244 HDTHFCGTIE258 YMAPEILMRS268 GHNRAVDWWS278 LGALMYDMLT288 GAPPFTGENR 298 KKTIDKILKC308 KLNLPPYLTQ318 EARDLLKKLL328 KRNAASRLGA338 GPGDAGEVQA 348 HPFFRHINWE358 ELLARKVEPP368 FKPLLQ
|
|||||
|
|
LEU97
3.784
GLY98
3.559
LYS99
3.544
GLY100
3.517
GLY101
4.452
TYR102
3.543
GLY103
3.385
LYS104
3.633
VAL105
3.807
ALA121
3.399
LYS123
3.345
VAL124
4.078
|
|||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|









| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| ErbB signaling pathway | hsa04012 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
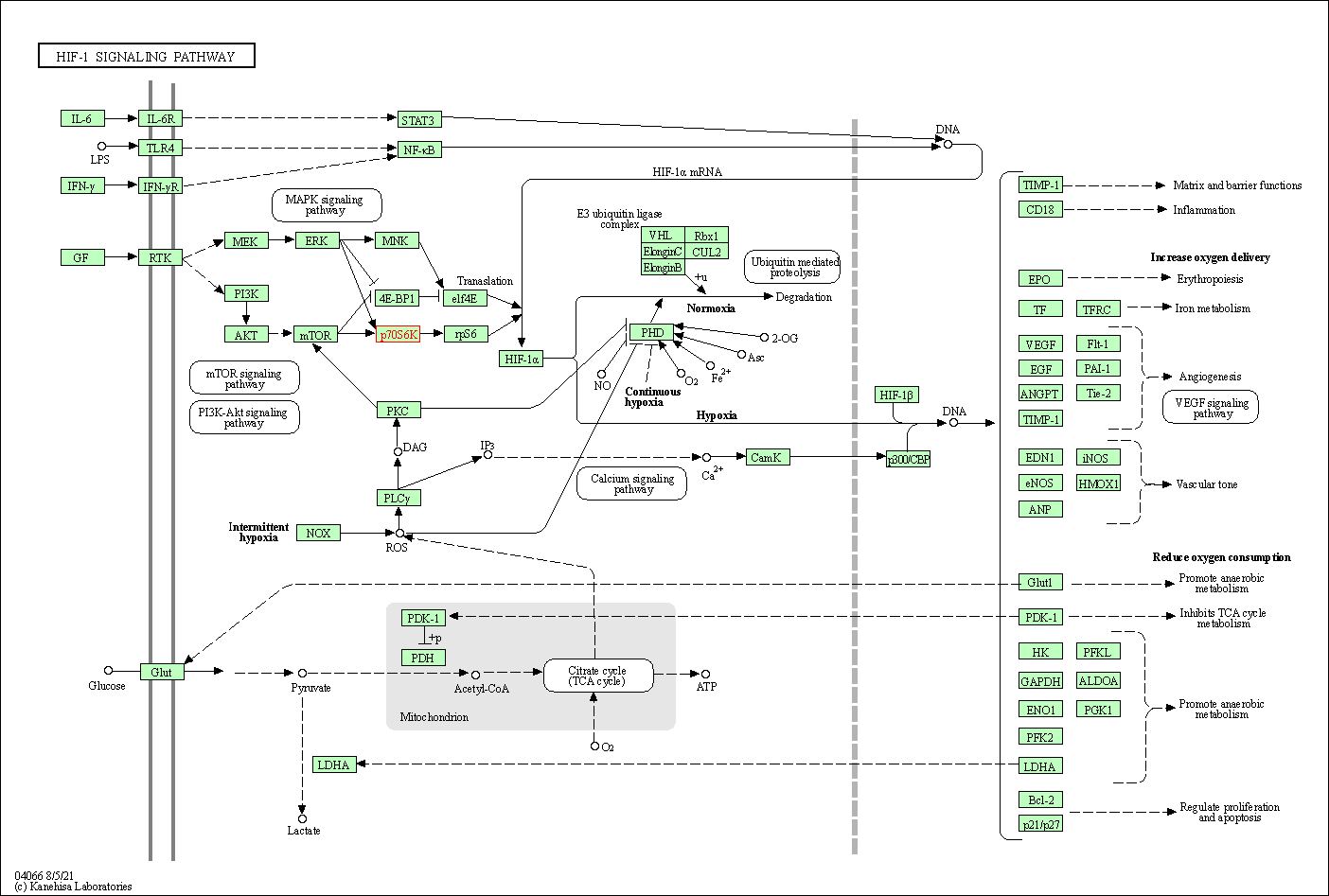
| HIF-1 signaling pathway | hsa04066 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Autophagy - animal | hsa04140 | Affiliated Target |

|
| Class: Cellular Processes => Transport and catabolism | Pathway Hierarchy | ||
| mTOR signaling pathway | hsa04150 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
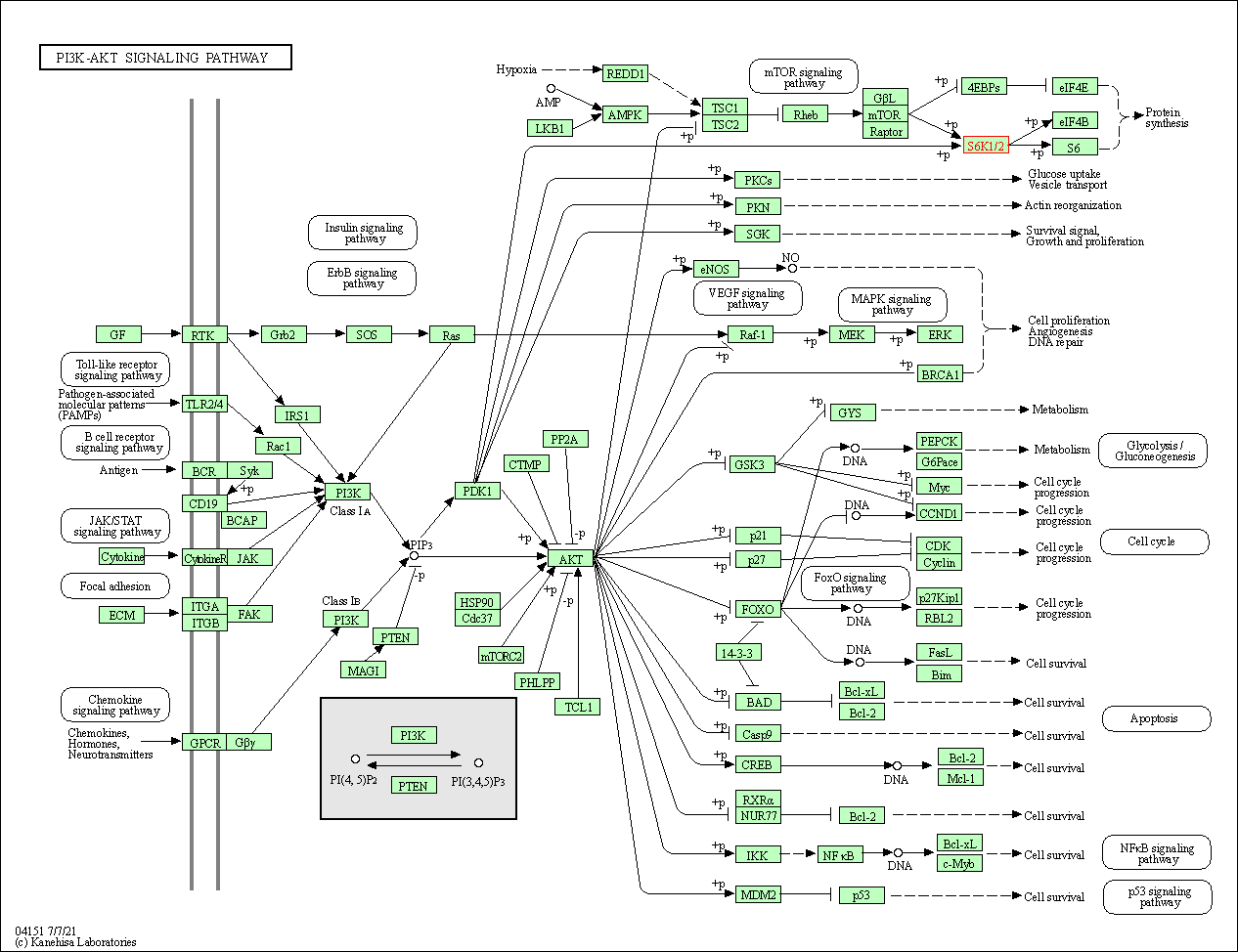
| PI3K-Akt signaling pathway | hsa04151 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| AMPK signaling pathway | hsa04152 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Longevity regulating pathway | hsa04211 | Affiliated Target |

|
| Class: Organismal Systems => Aging | Pathway Hierarchy | ||
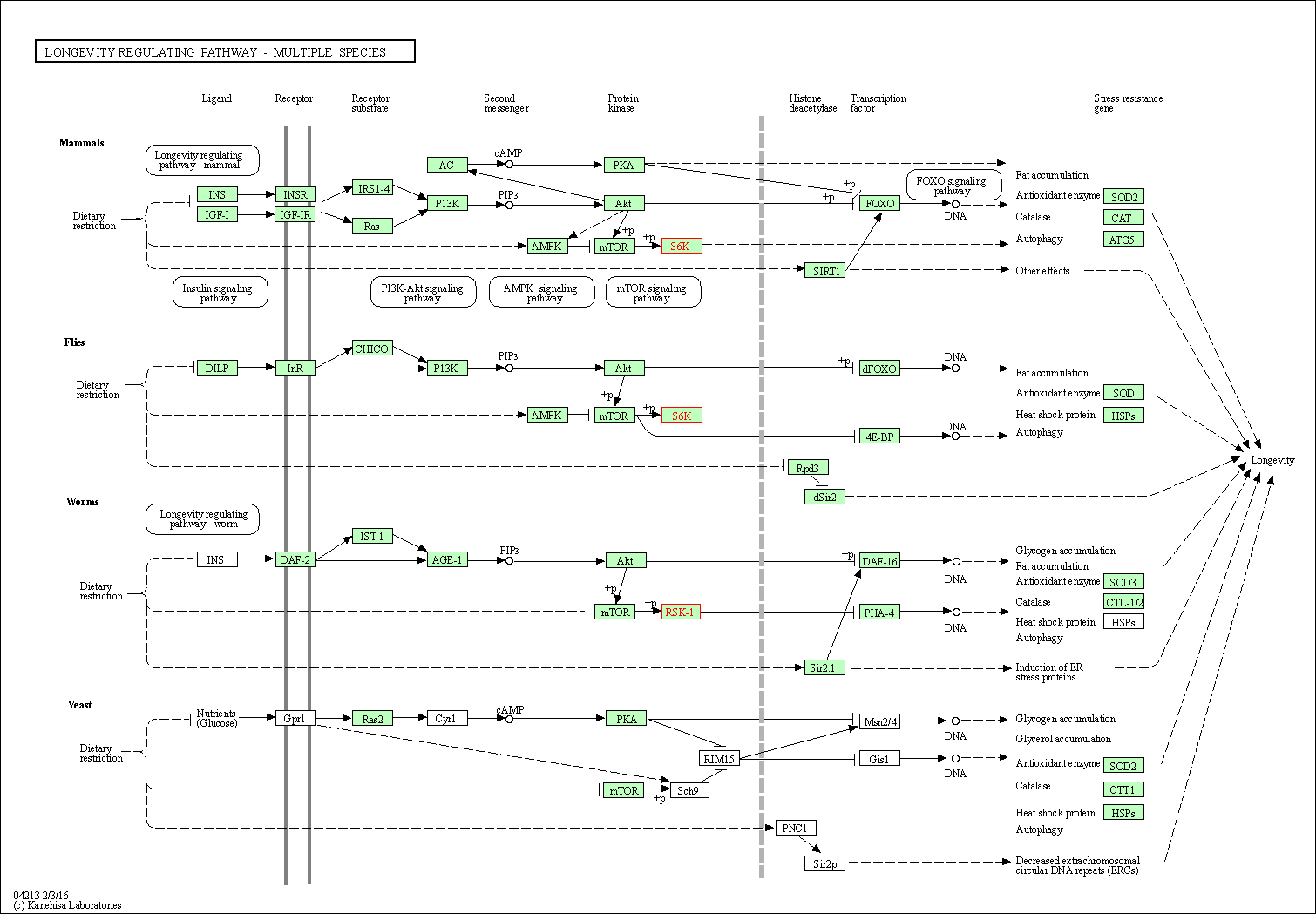
| Longevity regulating pathway - multiple species | hsa04213 | Affiliated Target |

|
| Class: Organismal Systems => Aging | Pathway Hierarchy | ||
| TGF-beta signaling pathway | hsa04350 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Apelin signaling pathway | hsa04371 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Fc gamma R-mediated phagocytosis | hsa04666 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Thermogenesis | hsa04714 | Affiliated Target |

|
| Class: Organismal Systems => Environmental adaptation | Pathway Hierarchy | ||
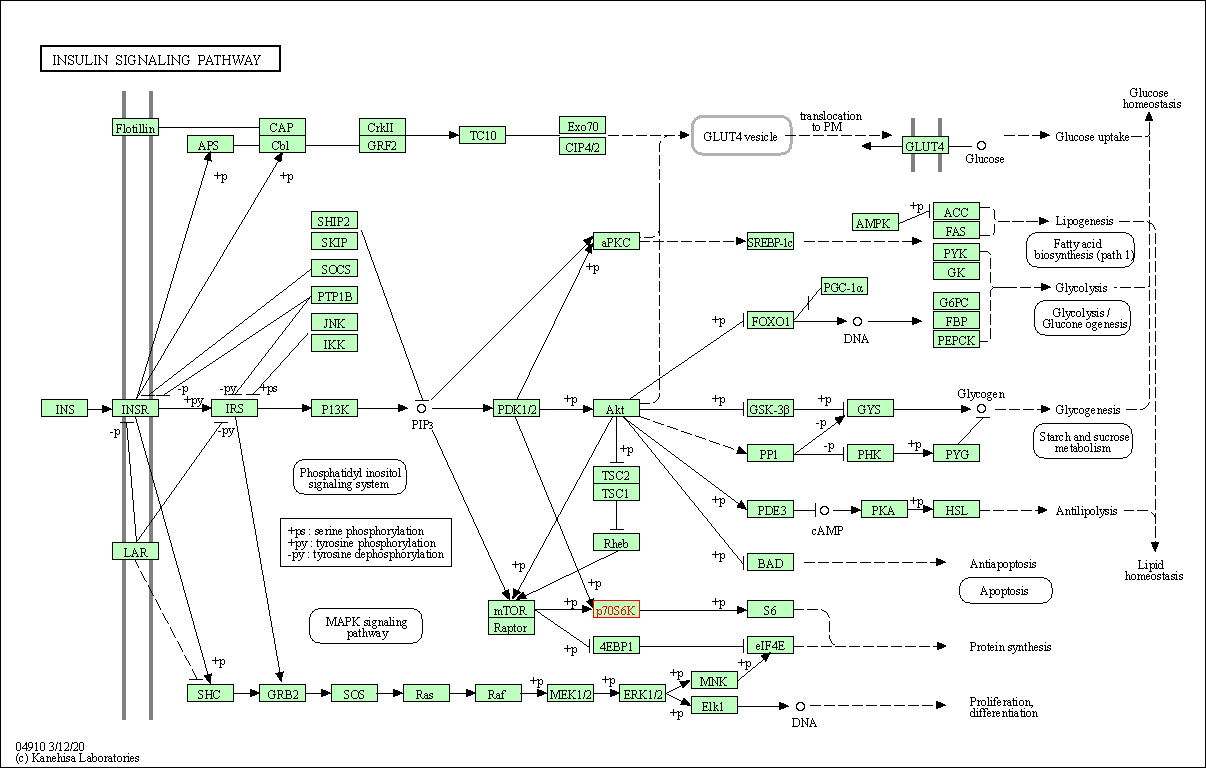
| Insulin signaling pathway | hsa04910 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 34 | Degree centrality | 3.65E-03 | Betweenness centrality | 3.87E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.59E-01 | Radiality | 1.45E+01 | Clustering coefficient | 1.98E-01 |
| Neighborhood connectivity | 5.11E+01 | Topological coefficient | 5.76E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000 Oct 1;351(Pt 1):95-105. | |||||
| REF 2 | ClinicalTrials.gov (NCT01971515) First-in-Human Dose Escalation Trial in Subjects With Advanced Malignancies. U.S. National Institutes of Health. | |||||
| REF 3 | ClinicalTrials.gov (NCT04074837) Phase1a, Randomized Placebo-controlled, Single and Multiple Dose, Dose-escalation Study to Evaluate the Safety, Tolerability and Pharmacokinetics of Oral NNI-362 in Healthy Aged Volunteers 50 to 72 Years of Age. U.S.National Institutes of Health. | |||||
| REF 4 | Characterization of PF-4708671, a novel and highly specific inhibitor of p70 ribosomal S6 kinase (S6K1). Biochem J. 2010 Oct 15;431(2):245-55. | |||||
| REF 5 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 6 | Novel pharmacotherapy: NNI-362, an allosteric p70S6 kinase stimulator, reverses cognitive and neural regenerative deficits in models of aging and disease. Stem Cell Res Ther. 2021 Jan 13;12(1):59. | |||||
| REF 7 | Ribosomal S6 kinase (RSK) modulators: a patent review.Expert Opin Ther Pat. 2016 Sep;26(9):1061-78. | |||||
| REF 8 | Optimization of protein kinase CK2 inhibitors derived from 4,5,6,7-tetrabromobenzimidazole. J Med Chem. 2004 Dec 2;47(25):6239-47. | |||||
| REF 9 | 4-arylazo-3,5-diamino-1H-pyrazole CDK inhibitors: SAR study, crystal structure in complex with CDK2, selectivity, and cellular effects. J Med Chem. 2006 Nov 2;49(22):6500-9. | |||||
| REF 10 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1525). | |||||
| REF 11 | 2,3,5-Trisubstituted pyridines as selective AKT inhibitors. Part II: Improved drug-like properties and kinase selectivity from azaindazoles. Bioorg Med Chem Lett. 2010 Jan 15;20(2):679-83. | |||||
| REF 12 | 3-Anilino-4-arylmaleimides: potent and selective inhibitors of glycogen synthase kinase-3 (GSK-3). Bioorg Med Chem Lett. 2001 Mar 12;11(5):635-9. | |||||
| REF 13 | (1H-imidazo[4,5-c]pyridin-2-yl)-1,2,5-oxadiazol-3-ylamine derivatives: further optimisation as highly potent and selective MSK-1-inhibitors. Bioorg Med Chem Lett. 2005 Jul 15;15(14):3407-11. | |||||
| REF 14 | Identification of Clinical Candidate M2698, a Dual p70S6K and Akt Inhibitor, for Treatment of PAM Pathway-Altered Cancers. J Med Chem. 2021 Oct 14;64(19):14603-14619. | |||||
| REF 15 | Crystal structures of the S6K1 kinase domain in complexes with inhibitors. J Struct Funct Genomics. 2014 Sep;15(3):153-64. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

