Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T51191
(Former ID: TTDR01156)
|
|||||
| Target Name |
Histone deacetylase 2 (HDAC2)
|
|||||
| Synonyms |
HD2
Click to Show/Hide
|
|||||
| Gene Name |
HDAC2
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Lymphoma [ICD-11: 2A80-2A86] | |||||
| 2 | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||||
| Function |
Gives a tag for epigenetic repression and plays an important role in transcriptional regulation, cell cycle progression and developmental events. Histone deacetylases act via the formation of large multiprotein complexes. Forms transcriptional repressor complexes by associating with MAD, SIN3, YY1 and N-COR. Interacts in the late S-phase of DNA-replication with DNMT1 in the other transcriptional repressor complex composed of DNMT1, DMAP1, PCNA, CAF1. Deacetylates TSHZ3 and regulates its transcriptional repressor activity. Component of a RCOR/GFI/KDM1A/HDAC complex that suppresses, via histone deacetylase (HDAC) recruitment, a number of genes implicated in multilineage blood cell development. May be involved in the transcriptional repression of circadian target genes, such as PER1, mediated by CRY1 through histone deacetylation. Involved in MTA1-mediated transcriptional corepression of TFF1 and CDKN1A. Responsible for the deacetylation of lysine residues on the N-terminal part of the core histones (H2A, H2B, H3 and H4).
Click to Show/Hide
|
|||||
| BioChemical Class |
Carbon-nitrogen hydrolase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 3.5.1.98
|
|||||
| Sequence |
MAYSQGGGKKKVCYYYDGDIGNYYYGQGHPMKPHRIRMTHNLLLNYGLYRKMEIYRPHKA
TAEEMTKYHSDEYIKFLRSIRPDNMSEYSKQMQRFNVGEDCPVFDGLFEFCQLSTGGSVA GAVKLNRQQTDMAVNWAGGLHHAKKSEASGFCYVNDIVLAILELLKYHQRVLYIDIDIHH GDGVEEAFYTTDRVMTVSFHKYGEYFPGTGDLRDIGAGKGKYYAVNFPMRDGIDDESYGQ IFKPIISKVMEMYQPSAVVLQCGADSLSGDRLGCFNLTVKGHAKCVEVVKTFNLPLLMLG GGGYTIRNVARCWTYETAVALDCEIPNELPYNDYFEYFGPDFKLHISPSNMTNQNTPEYM EKIKQRLFENLRMLPHAPGVQMQAIPEDAVHEDSGDEDGEDPDKRISIRASDKRIACDEE FSDSEDEGEGGRRNVADHKKGAKKARIEEDKKETEDKKTDVKEEDKSKDNSGEKTDTKGT KSEQLSNP Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| ADReCS ID | BADD_A02700 | |||||
| HIT2.0 ID | T68M1D | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 1 Clinical Trial Drugs | + | ||||
| 1 | CHR-3996 | Drug Info | Phase 1/2 | Lymphoma | [2] | |
| Mode of Action | [+] 1 Modes of Action | + | ||||
| Inhibitor | [+] 113 Inhibitor drugs | + | ||||
| 1 | CHR-3996 | Drug Info | [1] | |||
| 2 | PMID29671355-Compound-11 | Drug Info | [3] | |||
| 3 | PMID29671355-Compound-21 | Drug Info | [3] | |||
| 4 | PMID29671355-Compound-23 | Drug Info | [3] | |||
| 5 | PMID29671355-Compound-25 | Drug Info | [3] | |||
| 6 | PMID29671355-Compound-31 | Drug Info | [3] | |||
| 7 | PMID29671355-Compound-43 | Drug Info | [3] | |||
| 8 | PMID29671355-Compound-44 | Drug Info | [3] | |||
| 9 | PMID29671355-Compound-55 | Drug Info | [3] | |||
| 10 | PMID29671355-Compound-56 | Drug Info | [3] | |||
| 11 | PMID29671355-Compound-59 | Drug Info | [3] | |||
| 12 | PMID29671355-Compound-61 | Drug Info | [3] | |||
| 13 | PMID29671355-Compound-62 | Drug Info | [3] | |||
| 14 | PMID29671355-Compound-67 | Drug Info | [3] | |||
| 15 | PMID29671355-Compound-74 | Drug Info | [3] | |||
| 16 | PMID29671355-Compound-8 | Drug Info | [3] | |||
| 17 | PMID29671355-Compound-9 | Drug Info | [3] | |||
| 18 | (E)-8-Biphenyl-4-yl-1-oxazol-2-yl-oct-7-en-1-one | Drug Info | [4] | |||
| 19 | 1,1,1-Trifluoro-8-(4-phenoxy-phenoxy)-octan-2-one | Drug Info | [5] | |||
| 20 | 1,1,1-Trifluoro-8-phenoxy-octan-2-one | Drug Info | [5] | |||
| 21 | 2-(allyloxy)-N8-hydroxy-N1-phenyloctanediamide | Drug Info | [6] | |||
| 22 | 2-(benzyloxy)-N7-hydroxy-N1-phenylheptanediamide | Drug Info | [6] | |||
| 23 | 2-(methylsulfonylthio)ethyl 2-propylpentanoate | Drug Info | [7] | |||
| 24 | 4-Benzoylamino-N-hydroxy-benzamide | Drug Info | [8] | |||
| 25 | 4-Butyrylamino-N-hydroxy-benzamide | Drug Info | [9] | |||
| 26 | 4-Chloro-N-(5-hydroxycarbamoyl-pentyl)-benzamide | Drug Info | [10] | |||
| 27 | 4-Dimethylamino-N-(6-mercapto-hexyl)-benzamide | Drug Info | [11] | |||
| 28 | 4-Hydroxy-N-(5-hydroxycarbamoyl-pentyl)-benzamide | Drug Info | [12] | |||
| 29 | 4-Phenylbutyrohydroxamic acid | Drug Info | [13] | |||
| 30 | 5-(4-Chloro-phenyl)-pentanoic acid hydroxyamide | Drug Info | [14] | |||
| 31 | 5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione | Drug Info | [7] | |||
| 32 | 5-(Biphenyl-4-yl)-pentanoic acid N-hydroxyamide | Drug Info | [15] | |||
| 33 | 5-Mercapto-pentanoic acid phenylamide | Drug Info | [11] | |||
| 34 | 6-(2-Bromo-acetylamino)-hexanoic acid phenylamide | Drug Info | [11] | |||
| 35 | 6-benzenesulfinylhexanoic acid hydroxamide | Drug Info | [16] | |||
| 36 | 6-benzenesulfonylhexanoic acid hydroxamide | Drug Info | [16] | |||
| 37 | 6-Mercapto-hexanoic acid phenylamide | Drug Info | [11] | |||
| 38 | 6-Phenoxy-hexane-1-thiol | Drug Info | [11] | |||
| 39 | 6-phenylsulfanylhexanoic acid hydroxamide | Drug Info | [16] | |||
| 40 | 7-(Biphenyl-3-yloxy)-1-oxazol-2-yl-heptan-1-one | Drug Info | [4] | |||
| 41 | 7-(Biphenyl-4-yloxy)-1,1,1-trifluoro-heptan-2-one | Drug Info | [5] | |||
| 42 | 7-(Biphenyl-4-yloxy)-1-oxazol-2-yl-heptan-1-one | Drug Info | [4] | |||
| 43 | 7-(Biphenyl-4-yloxy)-heptanoic acid hydroxyamide | Drug Info | [17] | |||
| 44 | 7-(Naphthalen-2-yloxy)-1-oxazol-2-yl-heptan-1-one | Drug Info | [4] | |||
| 45 | 7-Biphenyl-4-yl-heptanoic acid hydroxyamide | Drug Info | [18] | |||
| 46 | 7-Mercapto-heptanoic acid benzothiazol-2-ylamide | Drug Info | [11] | |||
| 47 | 7-Mercapto-heptanoic acid biphenyl-3-ylamide | Drug Info | [11] | |||
| 48 | 7-Mercapto-heptanoic acid biphenyl-4-ylamide | Drug Info | [11] | |||
| 49 | 7-Mercapto-heptanoic acid phenylamide | Drug Info | [11] | |||
| 50 | 7-Mercapto-heptanoic acid pyridin-3-ylamide | Drug Info | [11] | |||
| 51 | 7-Mercapto-heptanoic acid quinolin-3-ylamide | Drug Info | [11] | |||
| 52 | 7-Phenoxy-heptanoic acid hydroxyamide | Drug Info | [18] | |||
| 53 | 8-(Biphenyl-3-yloxy)-1,1,1-trifluoro-octan-2-one | Drug Info | [5] | |||
| 54 | 8-(Biphenyl-4-yloxy)-1,1,1-trifluoro-octan-2-one | Drug Info | [5] | |||
| 55 | 8-(Biphenyl-4-yloxy)-2-oxo-octanoic acid | Drug Info | [17] | |||
| 56 | 8-Mercapto-octanoic acid phenylamide | Drug Info | [11] | |||
| 57 | 8-Oxo-8-phenyl-octanoic acid | Drug Info | [12] | |||
| 58 | 8-Oxo-8-phenyl-octanoic acid hydroxyamide | Drug Info | [10] | |||
| 59 | 8-Phenyl-octanoic acid hydroxyamide | Drug Info | [18] | |||
| 60 | 9,9,9-Trifluoro-8-oxo-nonanoic acid phenylamide | Drug Info | [5] | |||
| 61 | 9-(Biphenyl-4-yloxy)-1,1,1-trifluoro-nonan-2-one | Drug Info | [5] | |||
| 62 | Cyclostellettamine derivative | Drug Info | [19] | |||
| 63 | KAR-1880 | Drug Info | [20] | |||
| 64 | N-(2-amino-5-(benzofuran-2-yl)phenyl)benzamide | Drug Info | [21] | |||
| 65 | N-(2-amino-5-(furan-2-yl)phenyl)benzamide | Drug Info | [21] | |||
| 66 | N-(2-amino-5-(furan-3-yl)phenyl)benzamide | Drug Info | [21] | |||
| 67 | N-(2-amino-5-(pyridin-4-yl)phenyl)benzamide | Drug Info | [21] | |||
| 68 | N-(2-amino-5-(thiazol-2-yl)phenyl)benzamide | Drug Info | [21] | |||
| 69 | N-(2-amino-5-(thiophen-2-yl)phenyl)nicotinamide | Drug Info | [22] | |||
| 70 | N-(2-aminophenyl)-4-methoxybenzamide | Drug Info | [23] | |||
| 71 | N-(2-aminophenyl)benzamide | Drug Info | [21] | |||
| 72 | N-(2-aminophenyl)nicotinamide | Drug Info | [22] | |||
| 73 | N-(2-aminophenyl)quinoxaline-6-carboxamide | Drug Info | [23] | |||
| 74 | N-(2-Mercapto-ethyl)-N'-phenyl-oxalamide | Drug Info | [24] | |||
| 75 | N-(2-Mercapto-ethyl)-N'-phenyl-succinamide | Drug Info | [24] | |||
| 76 | N-(3'-acetyl-4-aminobiphenyl-3-yl)benzamide | Drug Info | [21] | |||
| 77 | N-(4'-acetyl-4-aminobiphenyl-3-yl)benzamide | Drug Info | [21] | |||
| 78 | N-(4-amino-3'-methoxybiphenyl-3-yl)benzamide | Drug Info | [21] | |||
| 79 | N-(4-amino-3'-methylbiphenyl-3-yl)benzamide | Drug Info | [21] | |||
| 80 | N-(4-amino-4'-bromobiphenyl-3-yl)benzamide | Drug Info | [21] | |||
| 81 | N-(4-amino-4'-fluorobiphenyl-3-yl)benzamide | Drug Info | [21] | |||
| 82 | N-(4-amino-4'-methoxybiphenyl-3-yl)benzamide | Drug Info | [21] | |||
| 83 | N-(4-amino-4'-vinylbiphenyl-3-yl)benzamide | Drug Info | [21] | |||
| 84 | N-(4-aminobiphenyl-3-yl)benzamide | Drug Info | [21] | |||
| 85 | N-(4-aminobiphenyl-3-yl)nicotinamide | Drug Info | [22] | |||
| 86 | N-(4-hydroxybiphenyl-3-yl)benzamide | Drug Info | [22] | |||
| 87 | N-(5-Hydroxycarbamoyl-pentyl)-4-nitro-benzamide | Drug Info | [10] | |||
| 88 | N-(6-Hydroxycarbamoyl-hexyl)-benzamide | Drug Info | [12] | |||
| 89 | N-(6-Mercapto-hexyl)-benzamide | Drug Info | [11] | |||
| 90 | N-Hydroxy-4-((R)-2-phenyl-butyrylamino)-benzamide | Drug Info | [8] | |||
| 91 | N-Hydroxy-4-((S)-2-phenyl-butyrylamino)-benzamide | Drug Info | [8] | |||
| 92 | N-Hydroxy-4-(2-phenyl-butyrylamino)-benzamide | Drug Info | [8] | |||
| 93 | N-Hydroxy-4-(3-phenyl-propionylamino)-benzamide | Drug Info | [25] | |||
| 94 | N-Hydroxy-4-(4-phenyl-butyrylamino)-benzamide | Drug Info | [8] | |||
| 95 | N-Hydroxy-4-(5-phenyl-pentanoylamino)-benzamide | Drug Info | [8] | |||
| 96 | N-Hydroxy-4-(pentanoylamino-methyl)-benzamide | Drug Info | [9] | |||
| 97 | N-Hydroxy-4-(phenylacetylamino-methyl)-benzamide | Drug Info | [9] | |||
| 98 | N-Hydroxy-4-phenylacetylamino-benzamide | Drug Info | [8] | |||
| 99 | N-Hydroxy-E-3-(4'-chlorobiphenyl-4-yl)-acrylamide | Drug Info | [15] | |||
| 100 | N-Hydroxy-E-3-(4'-cyanobiphenyl-4-yl)-acrylamide | Drug Info | [15] | |||
| 101 | N-Hydroxy-E-3-(biphenyl-4-yl)-acrylamide | Drug Info | [15] | |||
| 102 | N7-hydroxy-2-methoxy-N1-phenylheptanediamide | Drug Info | [6] | |||
| 103 | N7-hydroxy-N1-phenyl-2-propoxyheptanediamide | Drug Info | [6] | |||
| 104 | N8,2-dihydroxy-N1-phenyloctanediamide | Drug Info | [6] | |||
| 105 | N8-hydroxy-2-methoxy-N1-phenyloctanediamide | Drug Info | [6] | |||
| 106 | Octanedioic acid bis-hydroxyamide | Drug Info | [26] | |||
| 107 | Octanedioic acid hydroxyamide pyridin-2-ylamide | Drug Info | [12] | |||
| 108 | Octanedioic acid hydroxyamide pyridin-4-ylamide | Drug Info | [12] | |||
| 109 | PSAMMAPLIN A | Drug Info | [10] | |||
| 110 | santacruzamate A | Drug Info | [27] | |||
| 111 | ST-2986 | Drug Info | [28] | |||
| 112 | ST-2987 | Drug Info | [28] | |||
| 113 | Thioacetic acid S-(6-phenylcarbamoyl-hexyl) ester | Drug Info | [11] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Vorinostat | Ligand Info | |||||
| Structure Description | Structure of Human HDAC2 in complex with SAHA (vorinostat) | PDB:4LXZ | ||||
| Method | X-ray diffraction | Resolution | 1.85 Å | Mutation | No | [29] |
| PDB Sequence |
GKKKVCYYYD
21 GDIGNYYYGQ31 GHPMKPHRIR41 MTHNLLLNYG51 LYRKMEIYRP61 HKATAEEMTK 71 YHSDEYIKFL81 RSIRPDNMSE91 YSKQMQRFNV101 GEDCPVFDGL111 FEFCQLSTGG 121 SVAGAVKLNR131 QQTDMAVNWA141 GGLHHAKKSE151 ASGFCYVNDI161 VLAILELLKY 171 HQRVLYIDID181 IHHGDGVEEA191 FYTTDRVMTV201 SFHKYGEYFP211 GTGDLRDIGA 221 GKGKYYAVNF231 PMRDGIDDES241 YGQIFKPIIS251 KVMEMYQPSA261 VVLQCGADSL 271 SGDRLGCFNL281 TVKGHAKCVE291 VVKTFNLPLL301 MLGGGGYTIR311 NVARCWTYET 321 AVALDCEIPN331 ELPYNDYFEY341 FGPDFKLHIS351 PSNMTNQNTP361 EYMEKIKQRL 371 FENLRMLP
|
|||||
|
|
||||||
| Ligand Name: 1-Methoxy-2-[2-(2-Methoxy-Ethoxy]-Ethane | Ligand Info | |||||
| Structure Description | HDAC2 WITH LIGAND BRD4884 | PDB:5IWG | ||||
| Method | X-ray diffraction | Resolution | 1.66 Å | Mutation | No | [30] |
| PDB Sequence |
GKKKVCYYYD
21 GDIGNYYYGQ31 GHPMKPHRIR41 MTHNLLLNYG51 LYRKMEIYRP61 HKATAEEMTK 71 YHSDEYIKFL81 RSIRPDNMSE91 YSKQMQRFNV101 GEDCPVFDGL111 FEFCQLSTGG 121 SVAGAVKLNR131 QQTDMAVNWA141 GGLHHAKKSE151 ASGFCYVNDI161 VLAILELLKY 171 HQRVLYIDID181 IHHGDGVEEA191 FYTTDRVMTV201 SFHKYGEYFP211 GTGDLRDIGA 221 GKGKYYAVNF231 PMRDGIDDES241 YGQIFKPIIS251 KVMEMYQPSA261 VVLQCGADSL 271 SGDRLGCFNL281 TVKGHAKCVE291 VVKTFNLPLL301 MLGGGGYTIR311 NVARCWTYET 321 AVALDCEIPN331 ELPYNDYFEY341 FGPDFKLHIS351 PSNMTNQNTP361 EYMEKIKQRL 371 FENLRMLP
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|



| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
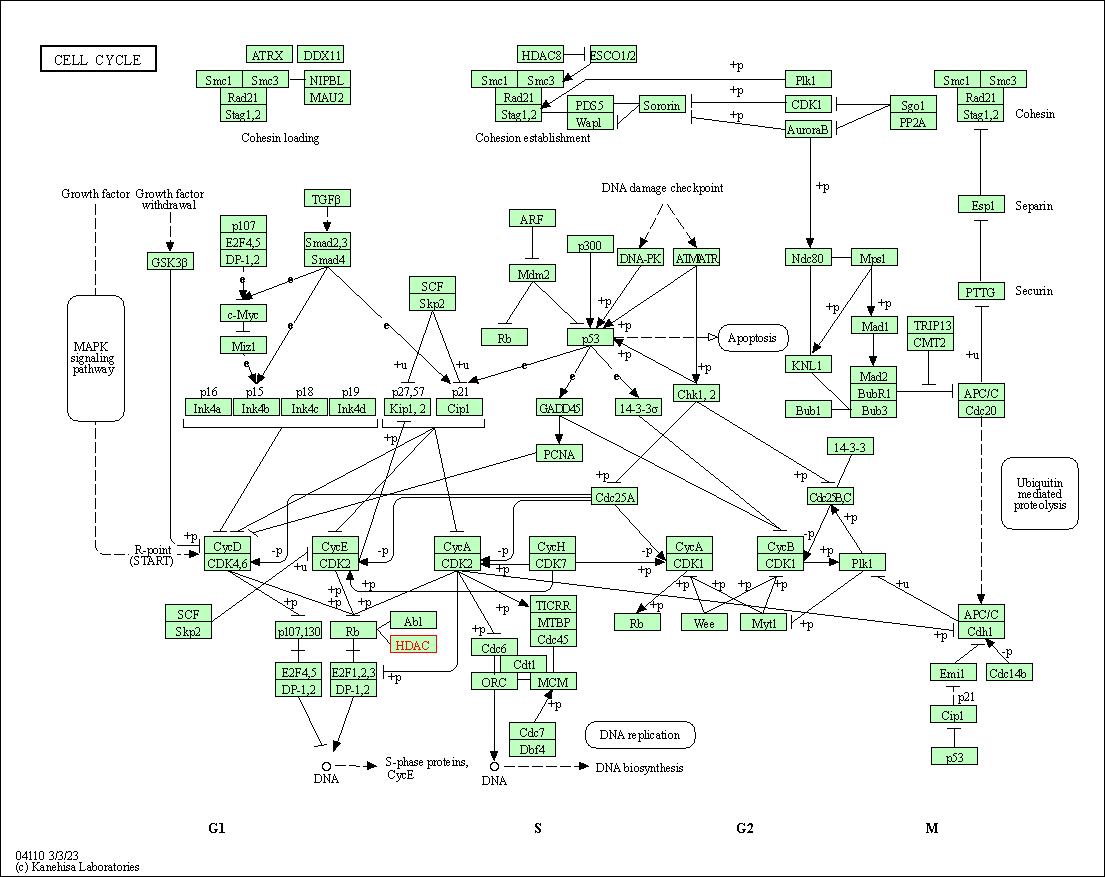
| Cell cycle | hsa04110 | Affiliated Target |

|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Longevity regulating pathway - multiple species | hsa04213 | Affiliated Target |

|
| Class: Organismal Systems => Aging | Pathway Hierarchy | ||
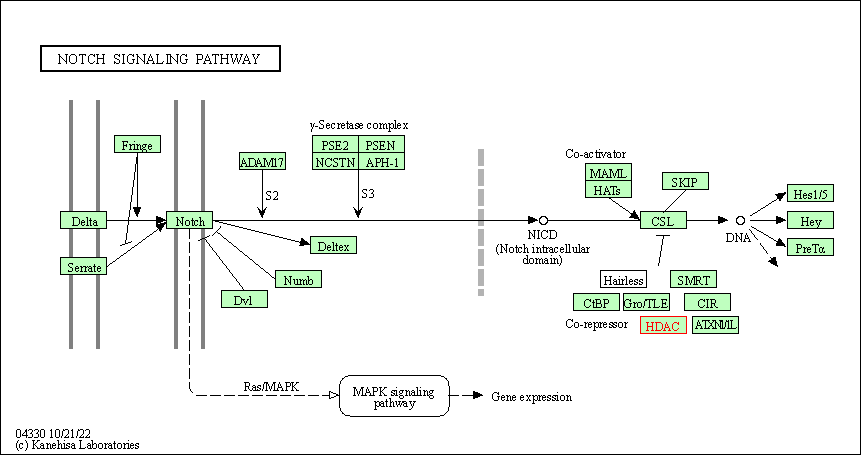
| Notch signaling pathway | hsa04330 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Neutrophil extracellular trap formation | hsa04613 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Thyroid hormone signaling pathway | hsa04919 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Degree | 77 | Degree centrality | 8.27E-03 | Betweenness centrality | 4.08E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.60E-01 | Radiality | 1.45E+01 | Clustering coefficient | 1.17E-01 |
| Neighborhood connectivity | 3.45E+01 | Topological coefficient | 3.31E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| Target QSAR Model | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | A phase I pharmacokinetic and pharmacodynamic study of CHR-3996, an oral class I selective histone deacetylase inhibitor in refractory solid tumors. Clin Cancer Res. 2012 May 1;18(9):2687-94. | |||||
| REF 2 | ClinicalTrials.gov (NCT03397706) Dose Escalation & Expansion Study of Oral VRx-3996 & Valganciclovir in Subjects With EBV-Associated Lymphoid Malignancies. U.S. National Institutes of Health. | |||||
| REF 3 | HDAC inhibitors: a 2013-2017 patent survey.Expert Opin Ther Pat. 2018 Apr 19:1-17. | |||||
| REF 4 | Heterocyclic ketones as inhibitors of histone deacetylase. Bioorg Med Chem Lett. 2003 Nov 17;13(22):3909-13. | |||||
| REF 5 | Trifluoromethyl ketones as inhibitors of histone deacetylase. Bioorg Med Chem Lett. 2002 Dec 2;12(23):3443-7. | |||||
| REF 6 | Omega-alkoxy analogues of SAHA (vorinostat) as inhibitors of HDAC: a study of chain-length and stereochemical dependence. Bioorg Med Chem Lett. 2007 Nov 15;17(22):6261-5. | |||||
| REF 7 | New sulfurated derivatives of valproic acid with enhanced histone deacetylase inhibitory activity. Bioorg Med Chem Lett. 2008 Mar 15;18(6):1893-7. | |||||
| REF 8 | Structure-based optimization of phenylbutyrate-derived histone deacetylase inhibitors. J Med Chem. 2005 Aug 25;48(17):5530-5. | |||||
| REF 9 | Zn2+-chelating motif-tethered short-chain fatty acids as a novel class of histone deacetylase inhibitors. J Med Chem. 2004 Jan 15;47(2):467-74. | |||||
| REF 10 | Histone deacetylase inhibitors. J Med Chem. 2003 Nov 20;46(24):5097-116. | |||||
| REF 11 | Novel inhibitors of human histone deacetylases: design, synthesis, enzyme inhibition, and cancer cell growth inhibition of SAHA-based non-hydroxama... J Med Chem. 2005 Feb 24;48(4):1019-32. | |||||
| REF 12 | Inhibitors of human histone deacetylase: synthesis and enzyme and cellular activity of straight chain hydroxamates. J Med Chem. 2002 Feb 14;45(4):753-7. | |||||
| REF 13 | Chemical phylogenetics of histone deacetylases. Nat Chem Biol. 2010 Mar;6(3):238-243. | |||||
| REF 14 | Stereodefined and polyunsaturated inhibitors of histone deacetylase based on (2E,4E)-5-arylpenta-2,4-dienoic acid hydroxyamides. Bioorg Med Chem Lett. 2004 May 17;14(10):2477-81. | |||||
| REF 15 | Design, synthesis, and evaluation of biphenyl-4-yl-acrylohydroxamic acid derivatives as histone deacetylase (HDAC) inhibitors. Eur J Med Chem. 2009 May;44(5):1900-12. | |||||
| REF 16 | Aromatic sulfide inhibitors of histone deacetylase based on arylsulfinyl-2,4-hexadienoic acid hydroxyamides. J Med Chem. 2006 Jan 26;49(2):800-5. | |||||
| REF 17 | Alpha-keto amides as inhibitors of histone deacetylase. Bioorg Med Chem Lett. 2003 Oct 6;13(19):3331-5. | |||||
| REF 18 | A novel series of histone deacetylase inhibitors incorporating hetero aromatic ring systems as connection units. Bioorg Med Chem Lett. 2003 Nov 3;13(21):3817-20. | |||||
| REF 19 | Three new cyclostellettamines, which inhibit histone deacetylase, from a marine sponge of the genus Xestospongia. Bioorg Med Chem Lett. 2004 May 17;14(10):2617-20. | |||||
| REF 20 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 2616). | |||||
| REF 21 | Exploration of the HDAC2 foot pocket: Synthesis and SAR of substituted N-(2-aminophenyl)benzamides. Bioorg Med Chem Lett. 2010 May 15;20(10):3142-5. | |||||
| REF 22 | Bioorg Med Chem Lett. 2008 Dec 1;18(23):6104-9. Epub 2008 Oct 14.SAR profiles of spirocyclic nicotinamide derived selective HDAC1/HDAC2 inhibitors (SHI-1:2). | |||||
| REF 23 | Novel aminophenyl benzamide-type histone deacetylase inhibitors with enhanced potency and selectivity. J Med Chem. 2007 Nov 15;50(23):5543-6. | |||||
| REF 24 | Mercaptoamide-based non-hydroxamic acid type histone deacetylase inhibitors. Bioorg Med Chem Lett. 2005 Apr 15;15(8):1969-72. | |||||
| REF 25 | Design, synthesis and preliminary biological evaluation of N-hydroxy-4-(3-phenylpropanamido)benzamide (HPPB) derivatives as novel histone deacetyla... Eur J Med Chem. 2009 Nov;44(11):4470-6. | |||||
| REF 26 | Structure-activity relationships on phenylalanine-containing inhibitors of histone deacetylase: in vitro enzyme inhibition, induction of differenti... J Med Chem. 2002 Jul 18;45(15):3296-309. | |||||
| REF 27 | Santacruzamate A, a potent and selective histone deacetylase inhibitor from the Panamanian marine cyanobacterium cf. Symploca sp. J Nat Prod. 2013 Nov 22;76(11):2026-33. | |||||
| REF 28 | N-Hydroxy-(4-oxime)-cinnamide: a versatile scaffold for the synthesis of novel histone deacetylase [correction of deacetilase] (HDAC) inhibitors. Bioorg Med Chem Lett. 2009 Apr 15;19(8):2346-9. | |||||
| REF 29 | Histone deacetylase (HDAC) inhibitor kinetic rate constants correlate with cellular histone acetylation but not transcription and cell viability. J Biol Chem. 2013 Sep 13;288(37):26926-43. | |||||
| REF 30 | Kinetic and structural insights into the binding of histone deacetylase 1 and 2 (HDAC1, 2) inhibitors. Bioorg Med Chem. 2016 Sep 15;24(18):4008-4015. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

