Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T27819
(Former ID: TTDR00754)
|
|||||
| Target Name |
Mitotic checkpoint protein (MAD1L1)
|
|||||
| Synonyms |
hMAD1; Tax-binding protein 181; TXBP181; Mitotic spindle assembly checkpoint protein MAD1; Mitotic checkpoint gene hMAD1; Mitotic checkpoint MAD1 protein homolog; Mitotic arrest deficient 1-like protein 1; MAD1-like protein 1; MAD1 (Mitotic arrest deficient, yeast, homolog)-like 1; MAD1; HsMAD1
Click to Show/Hide
|
|||||
| Gene Name |
MAD1L1
|
|||||
| Target Type |
Literature-reported target
|
[1] | ||||
| Function |
May recruit MAD2L1 to unattached kinetochores. Has a role in the correct positioning of the septum. Required for anchoring MAD2L1 to the nuclear periphery. Binds to the TERT promoter and represses telomerase expression, possibly by interfering with MYC binding. Component of the spindle-assembly checkpoint that prevents the onset of anaphase until all chromosomes are properly aligned at the metaphase plate.
Click to Show/Hide
|
|||||
| UniProt ID | ||||||
| Sequence |
MEDLGENTMVLSTLRSLNNFISQRVEGGSGLDISTSAPGSLQMQYQQSMQLEERAEQIRS
KSHLIQVEREKMQMELSHKRARVELERAASTSARNYEREVDRNQELLTRIRQLQEREAGA EEKMQEQLERNRQCQQNLDAASKRLREKEDSLAQAGETINALKGRISELQWSVMDQEMRV KRLESEKQELQEQLDLQHKKCQEANQKIQELQASQEARADHEQQIKDLEQKLSLQEQDAA IVKNMKSELVRLPRLERELKQLREESAHLREMRETNGLLQEELEGLQRKLGRQEKMQETL VGLELENERLLAKLQSWERLDQTMGLSIRTPEDLSRFVVELQQRELALKDKNSAVTSSAR GLEKARQQLQEELRQVSGQLLEERKKRETHEALARRLQKRVLLLTKERDGMRAILGSYDS ELTPAEYSPQLTRRMREAEDMVQKVHSHSAEMEAQLSQALEELGGQKQRADMLEMELKML KSQSSSAEQSFLFSREEADTLRLKVEELEGERSRLEEEKRMLEAQLERRALQGDYDQSRT KVLHMSLNPTSVARQRLREDHSQLQAECERLRGLLRAMERGGTVPADLEAAAASLPSSKE VAELKKQVESAELKNQRLKEVFQTKIQEFRKACYTLTGYQIDITTENQYRLTSLYAEHPG DCLIFKATSPSGSKMQLLETEFSHTVGELIEVHLRRQDSIPAFLSSLTLELFSRQTVA Click to Show/Hide
|
|||||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
| Protein Name | Pfam ID | Percentage of Identity (%) | E value |
|---|---|---|---|
| Protein Daple (CCDC88C) | 30.732 (63/205) | 2.00E-03 | |
| Laminin subunit alpha-2 (LAMA2) | 24.359 (57/234) | 5.00E-03 |
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cell cycle | hsa04110 | Affiliated Target |
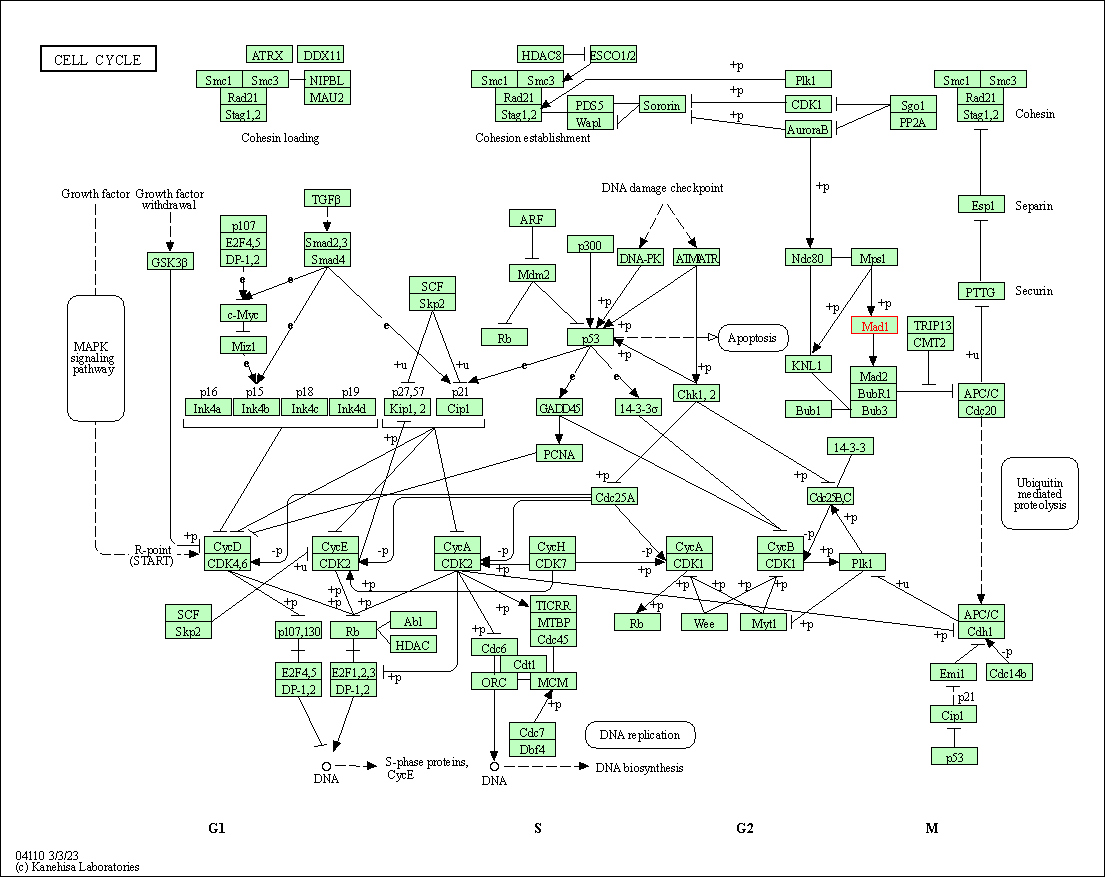
|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Oocyte meiosis | hsa04114 | Affiliated Target |
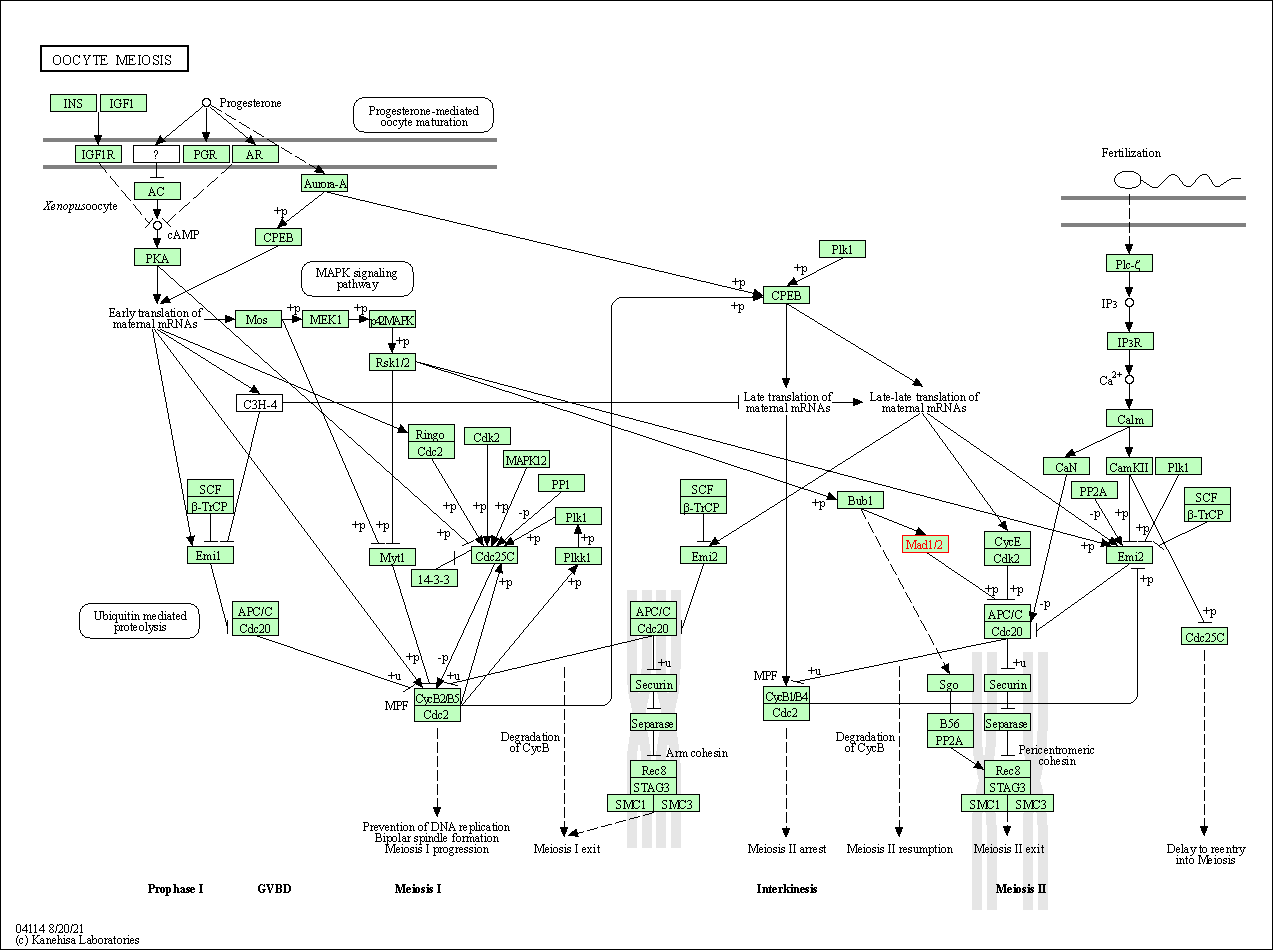
|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Progesterone-mediated oocyte maturation | hsa04914 | Affiliated Target |
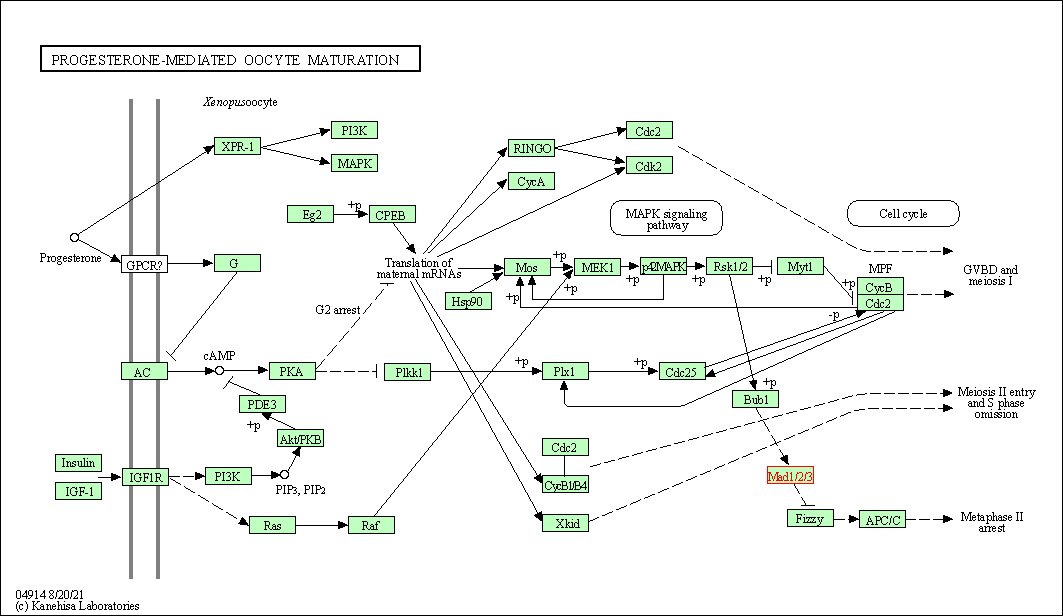
|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Degree | 7 | Degree centrality | 7.52E-04 | Betweenness centrality | 1.50E-05 |
|---|---|---|---|---|---|
| Closeness centrality | 1.92E-01 | Radiality | 1.33E+01 | Clustering coefficient | 5.24E-01 |
| Neighborhood connectivity | 3.49E+01 | Topological coefficient | 3.17E-01 | Eccentricity | 13 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-interacting Proteins | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Search for in vivo somatic mutations in the mitotic checkpoint gene, hMAD1, in human lung cancers. Oncogene. 1999 Nov 25;18(50):7180-3. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

