Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T02001
(Former ID: TTDS00499)
|
|||||
| Target Name |
Phosphodiesterase 4D (PDE4D)
|
|||||
| Synonyms |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D; PDE43; DPDE3
Click to Show/Hide
|
|||||
| Gene Name |
PDE4D
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Tonus and reflex abnormality [ICD-11: MB47] | |||||
| Function |
Hydrolyzes the second messenger cAMP, which is a key regulator of many important physiological processes.
Click to Show/Hide
|
|||||
| BioChemical Class |
Phosphoric diester hydrolase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 3.1.4.53
|
|||||
| Sequence |
MEAEGSSAPARAGSGEGSDSAGGATLKAPKHLWRHEQHHQYPLRQPQFRLLHPHHHLPPP
PPPSPQPQPQCPLQPPPPPPLPPPPPPPGAARGRYASSGATGRVRHRGYSDTERYLYCRA MDRTSYAVETGHRPGLKKSRMSWPSSFQGLRRFDVDNGTSAGRSPLDPMTSPGSGLILQA NFVHSQRRESFLYRSDSDYDLSPKSMSRNSSIASDIHGDDLIVTPFAQVLASLRTVRNNF AALTNLQDRAPSKRSPMCNQPSINKATITEEAYQKLASETLEELDWCLDQLETLQTRHSV SEMASNKFKRMLNRELTHLSEMSRSGNQVSEFISNTFLDKQHEVEIPSPTQKEKEKKKRP MSQISGVKKLMHSSSLTNSSIPRFGVKTEQEDVLAKELEDVNKWGLHVFRIAELSGNRPL TVIMHTIFQERDLLKTFKIPVDTLITYLMTLEDHYHADVAYHNNIHAADVVQSTHVLLST PALEAVFTDLEILAAIFASAIHDVDHPGVSNQFLINTNSELALMYNDSSVLENHHLAVGF KLLQEENCDIFQNLTKKQRQSLRKMVIDIVLATDMSKHMNLLADLKTMVETKKVTSSGVL LLDNYSDRIQVLQNMVHCADLSNPTKPLQLYRQWTDRIMEEFFRQGDRERERGMEISPMC DKHNASVEKSQVGFIDYIVHPLWETWADLVHPDAQDILDTLEDNREWYQSTIPQSPSPAP DDPEEGRQGQTEKFQFELTLEEDGESDTEKDSGSQVEEDTSCSDSKTLCTQDSESTEIPL DEQVEEEAVGEEEESQPEACVIDDRSPDT Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T66FP0 | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 1 Approved Drugs | + | ||||
| 1 | Papaverine | Drug Info | Approved | Spasm | [2] | |
| Clinical Trial Drug(s) | [+] 20 Clinical Trial Drugs | + | ||||
| 1 | DENBUFYLLINE | Drug Info | Phase 3 | Cognitive impairment | [3] | |
| 2 | SOTB07 | Drug Info | Phase 3 | Asthma | [4] | |
| 3 | AN-2898 | Drug Info | Phase 2 | Atopic dermatitis | [5] | |
| 4 | AWD-12-281 | Drug Info | Phase 2 | Rhinitis | [6] | |
| 5 | BPN14770 | Drug Info | Phase 2 | Alzheimer disease | [7] | |
| 6 | CC-1088 | Drug Info | Phase 2 | Crohn disease | [8] | |
| 7 | GPD-1116 | Drug Info | Phase 2 | Asthma | [9] | |
| 8 | HT-0712 | Drug Info | Phase 2 | Cognitive impairment | [10] | |
| 9 | LIRIMILAST | Drug Info | Phase 2 | Chronic obstructive pulmonary disease | [11] | |
| 10 | MK-0873 | Drug Info | Phase 2 | Psoriasis vulgaris | [12] | |
| 11 | Oglemilast | Drug Info | Phase 2 | Asthma | [13] | |
| 12 | OX-914 | Drug Info | Phase 2 | Asthma | [14] | |
| 13 | Piclamilast | Drug Info | Phase 2 | Rheumatoid arthritis | [15] | |
| 14 | Revamilast | Drug Info | Phase 2 | Asthma | [16] | |
| 15 | TA-7906 | Drug Info | Phase 2 | Atopic dermatitis | [17] | |
| 16 | TOFIMILAST | Drug Info | Phase 2 | Chronic obstructive pulmonary disease | [18] | |
| 17 | AVE-8112 | Drug Info | Phase 1 | Parkinson disease | [19] | |
| 18 | GSK-356278 | Drug Info | Phase 1 | Huntington disease | [20] | |
| 19 | MEM-1414 | Drug Info | Phase 1 | Mood disorder | [21] | |
| 20 | Ronomilast | Drug Info | Phase 1 | Chronic obstructive pulmonary disease | [22] | |
| Discontinued Drug(s) | [+] 14 Discontinued Drugs | + | ||||
| 1 | CDP840 | Drug Info | Discontinued in Phase 2 | Chronic obstructive pulmonary disease | [23] | |
| 2 | CI-1018 | Drug Info | Discontinued in Phase 2 | Asthma | [24] | |
| 3 | Daxalipram | Drug Info | Discontinued in Phase 2 | Multiple sclerosis | [25] | |
| 4 | KW-4490 | Drug Info | Discontinued in Phase 2 | Asthma | [26] | |
| 5 | LAS-37779 | Drug Info | Discontinued in Phase 2 | Psoriasis vulgaris | [27] | |
| 6 | V-11294A | Drug Info | Discontinued in Phase 2 | Asthma | [28] | |
| 7 | D-4418 | Drug Info | Discontinued in Phase 1 | Cutaneous T-cell lymphoma | [29] | |
| 8 | SCH-351591 | Drug Info | Discontinued in Phase 1 | Chronic obstructive pulmonary disease | [30] | |
| 9 | YM-976 | Drug Info | Discontinued in Phase 1 | Asthma | [31], [32] | |
| 10 | D-22888 | Drug Info | Terminated | Allergy | [33] | |
| 11 | GW-3600 | Drug Info | Terminated | Asthma | [34] | |
| 12 | NIK-616 | Drug Info | Terminated | Chronic obstructive pulmonary disease | [35] | |
| 13 | TJN-598 | Drug Info | Terminated | Glomerulonephritis | [36] | |
| 14 | Torbafylline | Drug Info | Terminated | Peripheral vascular disease | [37] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Inhibitor | [+] 48 Inhibitor drugs | + | ||||
| 1 | Papaverine | Drug Info | [1] | |||
| 2 | DENBUFYLLINE | Drug Info | [38] | |||
| 3 | SOTB07 | Drug Info | [39] | |||
| 4 | AN-2898 | Drug Info | [40] | |||
| 5 | AWD-12-281 | Drug Info | [41] | |||
| 6 | BPN14770 | Drug Info | [42], [43] | |||
| 7 | CC-1088 | Drug Info | [44] | |||
| 8 | GPD-1116 | Drug Info | [9] | |||
| 9 | HT-0712 | Drug Info | [39] | |||
| 10 | LIRIMILAST | Drug Info | [45] | |||
| 11 | MK-0873 | Drug Info | [46] | |||
| 12 | Oglemilast | Drug Info | [47] | |||
| 13 | OX-914 | Drug Info | [48] | |||
| 14 | Piclamilast | Drug Info | [49] | |||
| 15 | Revamilast | Drug Info | [50] | |||
| 16 | TA-7906 | Drug Info | [51] | |||
| 17 | TOFIMILAST | Drug Info | [45] | |||
| 18 | AVE-8112 | Drug Info | [52] | |||
| 19 | GSK-356278 | Drug Info | [53] | |||
| 20 | MEM-1414 | Drug Info | [54] | |||
| 21 | Ronomilast | Drug Info | [55] | |||
| 22 | CDP840 | Drug Info | [56] | |||
| 23 | CI-1018 | Drug Info | [57] | |||
| 24 | Daxalipram | Drug Info | [58] | |||
| 25 | KW-4490 | Drug Info | [59] | |||
| 26 | LAS-37779 | Drug Info | [60] | |||
| 27 | V-11294A | Drug Info | [61] | |||
| 28 | D-4418 | Drug Info | [62] | |||
| 29 | SCH-351591 | Drug Info | [63] | |||
| 30 | YM-976 | Drug Info | [45] | |||
| 31 | GW-3600 | Drug Info | [65] | |||
| 32 | NIK-616 | Drug Info | [66] | |||
| 33 | TJN-598 | Drug Info | [36] | |||
| 34 | ZAPRINAST | Drug Info | [67] | |||
| 35 | 1-Butyl-3-methyl-3,7-dihydro-purine-2,6-dione | Drug Info | [68] | |||
| 36 | 1-Methyl-3-propyl-3,7-dihydro-purine-2,6-dione | Drug Info | [68] | |||
| 37 | ASP-3258 | Drug Info | [39] | |||
| 38 | CD-160130 | Drug Info | [39] | |||
| 39 | CHF-5480 | Drug Info | [39] | |||
| 40 | isobutylmethylxanthine | Drug Info | [1], [69] | |||
| 41 | KF-66490 | Drug Info | [70] | |||
| 42 | L-454560 | Drug Info | [71] | |||
| 43 | L-869298 | Drug Info | [72] | |||
| 44 | OCID-2987 | Drug Info | [39] | |||
| 45 | ROLIPRAM | Drug Info | [73] | |||
| 46 | RS-25344 | Drug Info | [74] | |||
| 47 | TAS-203 | Drug Info | [39] | |||
| 48 | UCB-101333-3 | Drug Info | [75] | |||
| Modulator | [+] 5 Modulator drugs | + | ||||
| 1 | D-22888 | Drug Info | [64] | |||
| 2 | Torbafylline | Drug Info | [37] | |||
| 3 | AL-59640 | Drug Info | [39] | |||
| 4 | CH-3697 | Drug Info | [39] | |||
| 5 | ZL-N-91 | Drug Info | [39] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Apremilast | Ligand Info | |||||
| Structure Description | Crystal structure of PDE4D catalytic domain in complex with Apremilast | PDB:7CBQ | ||||
| Method | X-ray diffraction | Resolution | 1.59 Å | Mutation | No | [76] |
| PDB Sequence |
TEQEDVLAKE
95 LEDVNKWGLH105 VFRIAELSGN115 RPLTVIMHTI125 FQERDLLKTF135 KIPVDTLITY 145 LMTLEDHYHA155 DVAYHNNIHA165 ADVVQSTHVL175 LSTPALEAVF185 TDLEILAAIF 195 ASAIHDVDHP205 GVSNQFLINT215 NSELALMYND225 SSVLENHHLA235 VGFKLLQEEN 245 CDIFQNLTKK255 QRQSLRKMVI265 DIVLATDMSK275 HMNLLADLKT285 MVETKKVTSS 295 GVLLLDNYSD305 RIQVLQNMVH315 CADLSNPTKP325 LQLYRQWTDR335 IMEEFFRQGD 345 RERERGMEIS355 PMCDKHNASV365 EKSQVGFIDY375 IVHPLWETWA385 DLVHPDAQDI 395 LDTLEDNREW405 YQSTIP
|
|||||
|
|
TYR159
3.752
HIS160
3.156
SER208
3.901
THR271
4.213
MET273
3.064
ASP318
3.392
LEU319
3.853
ASN321
3.666
TYR329
4.237
TRP332
3.984
THR333
3.770
|
|||||
| Ligand Name: Roflumilast | Ligand Info | |||||
| Structure Description | Catalytic Domain Of Human Phosphodiesterase 4D In Complex With Roflumilast | PDB:1XOQ | ||||
| Method | X-ray diffraction | Resolution | 1.83 Å | Mutation | No | [77] |
| PDB Sequence |
TEQEDVLAKE
95 LEDVNKWGLH105 VFRIAELSGN115 RPLTVIMHTI125 FQERDLLKTF135 KIPVDTLITY 145 LMTLEDHYHA155 DVAYHNNIHA165 ADVVQSTHVL175 LSTPALEAVF185 TDLEILAAIF 195 ASAIHDVDHP205 GVSNQFLINT215 NSELALMYND225 SSVLENHHLA235 VGFKLLQEEN 245 CDIFQNLTKK255 QRQSLRKMVI265 DIVLATDMSK275 HMNLLADLKT285 MVETKKVTSS 295 GVLLLDNYSD305 RIQVLQNMVH315 CADLSNPTKP325 LQLYRQWTDR335 IMEEFFRQGD 345 RERERGMEIS355 PMCDKHNASV365 EKSQVGFIDY375 IVHPLWETWA385 DLVHPDAQDI 395 LDTLEDNREW405 YQSTIP
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
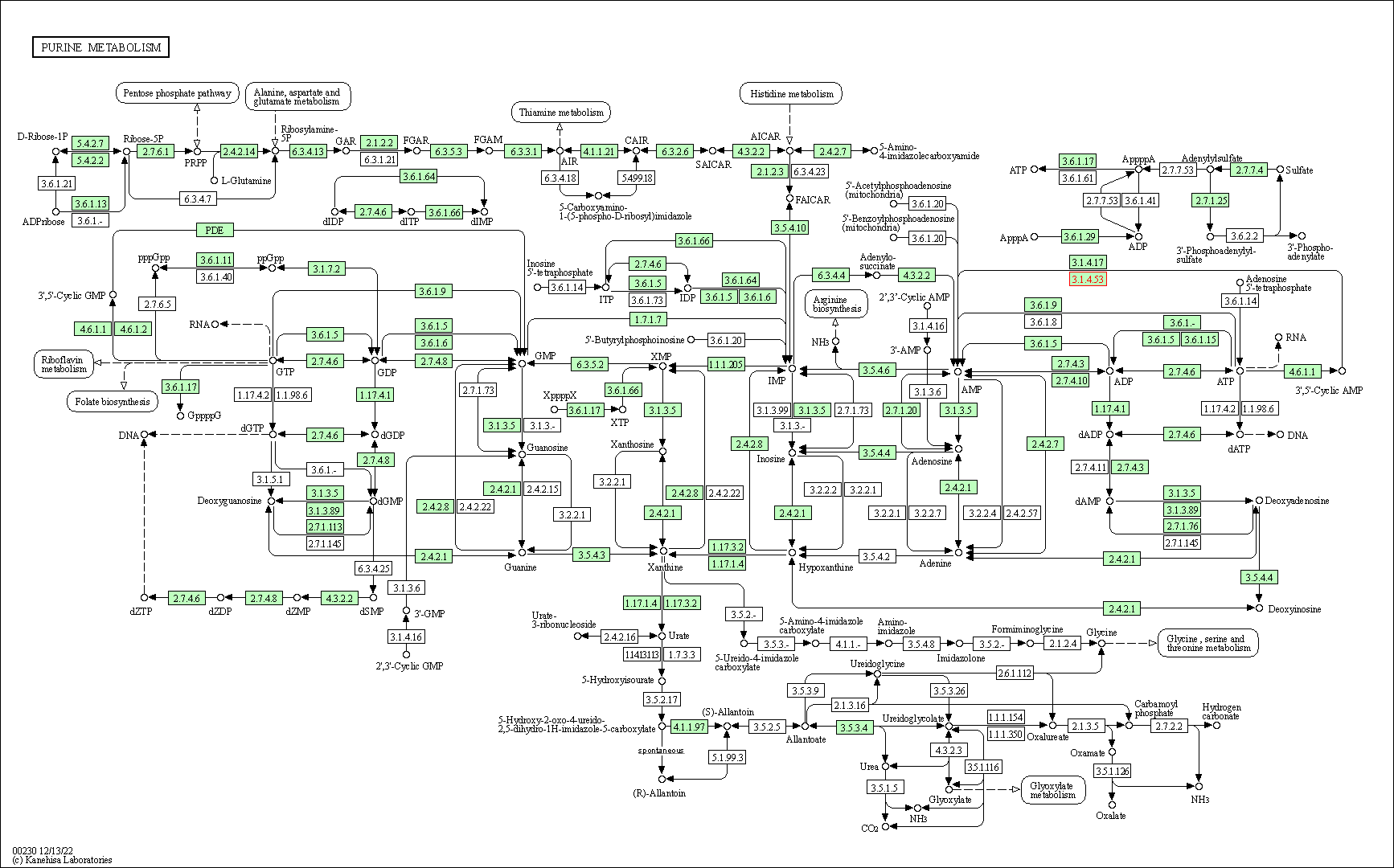
| Purine metabolism | hsa00230 | Affiliated Target |

|
| Class: Metabolism => Nucleotide metabolism | Pathway Hierarchy | ||
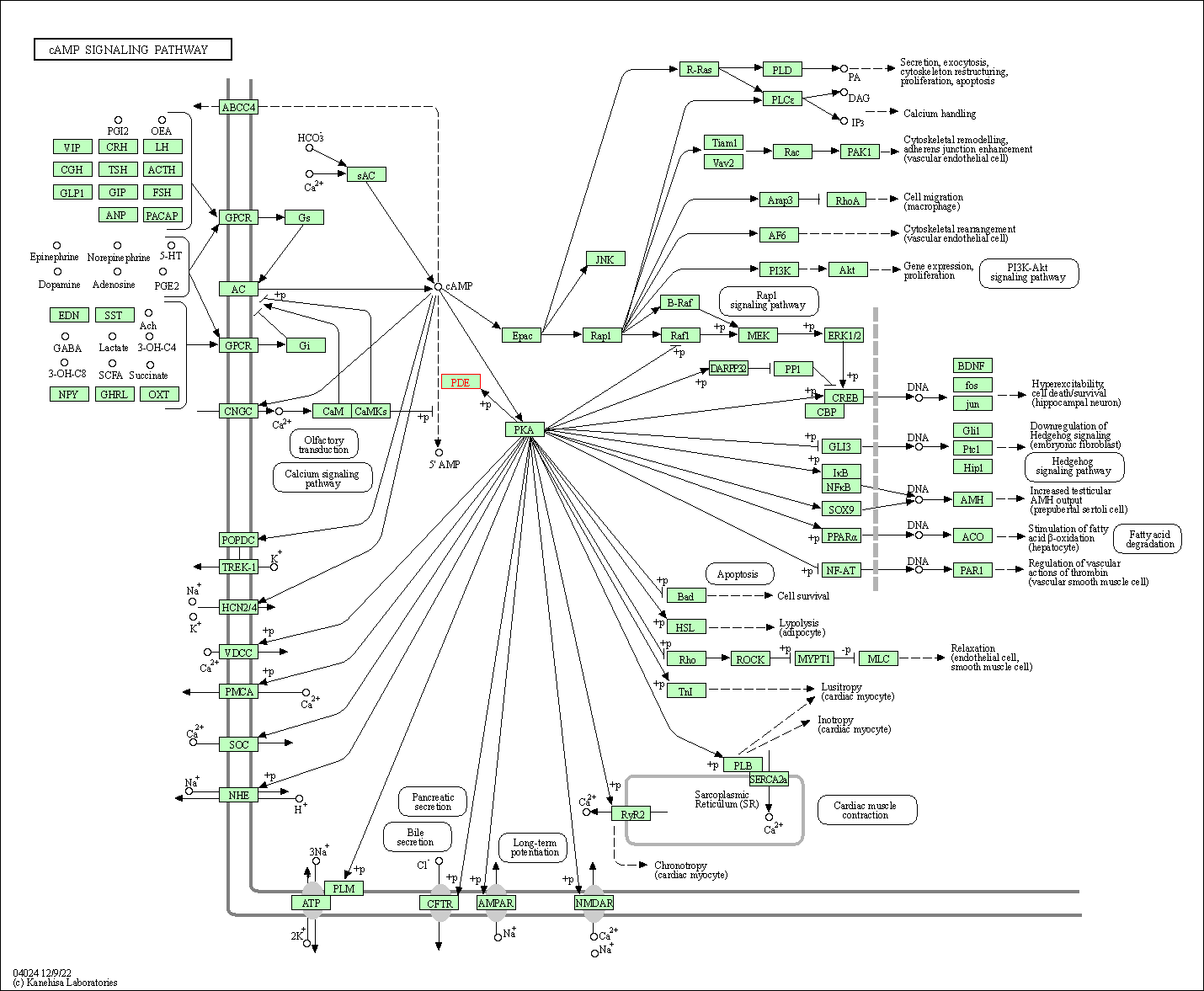
| cAMP signaling pathway | hsa04024 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
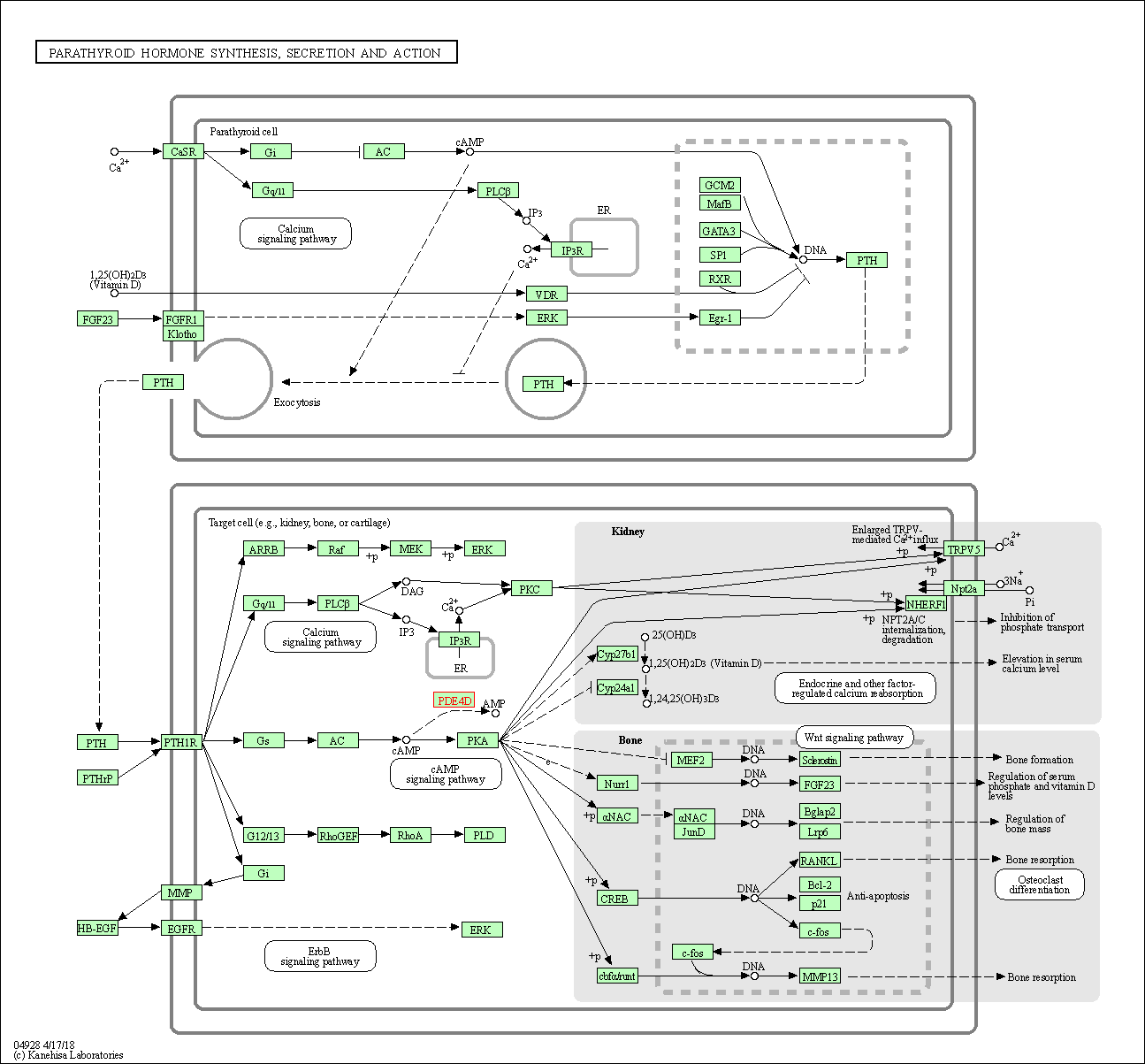
| Parathyroid hormone synthesis, secretion and action | hsa04928 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Degree | 3 | Degree centrality | 3.22E-04 | Betweenness centrality | 3.91E-05 |
|---|---|---|---|---|---|
| Closeness centrality | 2.09E-01 | Radiality | 1.37E+01 | Clustering coefficient | 0.00E+00 |
| Neighborhood connectivity | 2.37E+01 | Topological coefficient | 3.33E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 3 KEGG Pathways | + | ||||
| 1 | Purine metabolism | |||||
| 2 | cAMP signaling pathway | |||||
| 3 | Morphine addiction | |||||
| Pathwhiz Pathway | [+] 1 Pathwhiz Pathways | + | ||||
| 1 | Purine Metabolism | |||||
| Reactome | [+] 2 Reactome Pathways | + | ||||
| 1 | DARPP-32 events | |||||
| 2 | G alpha (s) signalling events | |||||
| WikiPathways | [+] 3 WikiPathways | + | ||||
| 1 | G Protein Signaling Pathways | |||||
| 2 | Myometrial Relaxation and Contraction Pathways | |||||
| 3 | TSH signaling pathway | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||||
| REF 2 | Drug information of Papaverine, 2008. eduDrugs. | |||||
| REF 3 | Denbufylline in dementia: a double-blind controlled study. Dement Geriatr Cogn Disord. 1999 Nov-Dec;10(6):505-10. | |||||
| REF 4 | ClinicalTrials.gov (NCT01907763) Study Phase III Study to Assess the Efficacy and Safety of SOTB07 in Asthma Patients. U.S. National Institutes of Health. | |||||
| REF 5 | ClinicalTrials.gov (NCT01301508) Efficacy and Safety of AN2898 and AN2728 Topical Ointments to Treat Mild-to-Moderate Atopic Dermatitis. U.S. National Institutes of Health. | |||||
| REF 6 | ClinicalTrials.gov (NCT00354510) Topical GW842470X In Adults Patients With Moderate Atopic Dermatitis. U.S. National Institutes of Health. | |||||
| REF 7 | ClinicalTrials.gov (NCT03817684) Tetra PICASSO AD Trial: Study to Evaluate Effects of BPN14770 in Early Alzheimer's Subjects. U.S. National Institutes of Health. | |||||
| REF 8 | ClinicalTrials.gov (NCT00045786) Study to Determine the Safety and Preliminary Efficacy of CC-1088 in the Treatment of Myelodysplastic Syndromes. U.S. National Institutes of Health. | |||||
| REF 9 | Phosphodiesterase 4 inhibitor GPD-1116 markedly attenuates the development of cigarette smoke-induced emphysema in senescence-accelerated mice P1 s... Am J Physiol Lung Cell Mol Physiol. 2008 Feb;294(2):L196-204. | |||||
| REF 10 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800018324) | |||||
| REF 11 | A novel phosphodiesterase 4 inhibitor template. Expert Opinion on Therapeutic Patents,2003, 13(6), 929-933. | |||||
| REF 12 | ClinicalTrials.gov (NCT00132730) An Investigational Drug Study In Patients With COPD (Chronic Obstructive Pulmonary Disease) (MK-0873-005). U.S. National Institutes of Health. | |||||
| REF 13 | ClinicalTrials.gov (NCT00671073) Study To Assess Efficacy and Safety of Oglemilast in Patients With Chronic Obstructive Pulmonary Disease (COPD). U.S. National Institutes of Health. | |||||
| REF 14 | ClinicalTrials.gov (NCT00758446) Efficacy and Safety Study of BLX-028914 in Subjects With Allergic Rhinitis. U.S. National Institutes of Health. | |||||
| REF 15 | Effect of food and gender on the pharmacokinetics of RP 73401, a phosphodiesterase IV inhibitor. Int J Clin Pharmacol Ther. 2000 Dec;38(12):588-94. | |||||
| REF 16 | ClinicalTrials.gov (NCT01436890) A Clinical Trial to Study the Effects of Revamilast in Patients With Chronic Persistent Asthma. U.S. National Institutes of Health. | |||||
| REF 17 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800024588) | |||||
| REF 18 | ClinicalTrials.gov (NCT00150397) A Study of the Safety and Efficacy of Tofimilast in Adult Asthmatics. U.S. National Institutes of Health. | |||||
| REF 19 | ClinicalTrials.gov (NCT01803945) A Multiple Ascending Dose Study to Assess the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of AVE8112 in Patients With Parkinson's Disease. U.S. National Institutes of Health. | |||||
| REF 20 | ClinicalTrials.gov (NCT01031186) First Time in Human Study. U.S. National Institutes of Health. | |||||
| REF 21 | The Quest for Human Longevity, Lewis D. Solo. Page(145). | |||||
| REF 22 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800013990) | |||||
| REF 23 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003087) | |||||
| REF 24 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010034) | |||||
| REF 25 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800015672) | |||||
| REF 26 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800017153) | |||||
| REF 27 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800022894) | |||||
| REF 28 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800012597) | |||||
| REF 29 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007757) | |||||
| REF 30 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010874) | |||||
| REF 31 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5292). | |||||
| REF 32 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010783) | |||||
| REF 33 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007179) | |||||
| REF 34 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800006447) | |||||
| REF 35 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800020194) | |||||
| REF 36 | Effects of TJN-598, a new selective phosphodiesterase type IV inhibitor on anti-Thy1 nephritis in rats. Clin Exp Nephrol. 2011 Feb;15(1):14-24. | |||||
| REF 37 | Phosphodiesterase (PDE) inhibitor torbafylline (HWA 448) attenuates burn-induced rat skeletal muscle proteolysis through the PDE4/cAMP/EPAC/PI3K/Akt pathway. Mol Cell Endocrinol. 2014 Aug 5;393(1-2):152-63. | |||||
| REF 38 | Pyrazolopyrimidine-2,4-dione sulfonamides: novel and selective calcitonin inducers. J Med Chem. 2002 May 23;45(11):2342-5. | |||||
| REF 39 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1303). | |||||
| REF 40 | An assessment of the genetic toxicology of novel boron-containing therapeutic agents. Environ Mol Mutagen. 2013 Jun;54(5):338-46. | |||||
| REF 41 | The phosphodiesterase 4 inhibitor AWD 12-281 is active in a new guinea-pig model of allergic skin inflammation predictive of human skin penetration and suppresses both Th1 and Th2 cytokines in mice. J Pharm Pharmacol. 2005 Dec;57(12):1609-17. | |||||
| REF 42 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 43 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 44 | Thalidomide analogs and PDE4 inhibition. Bioorg Med Chem Lett. 1998 Oct 6;8(19):2669-74. | |||||
| REF 45 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 46 | MK-0873, a PDE4 inhibitor, does not influence the pharmacokinetics of theophylline in healthy male volunteers. Pulm Pharmacol Ther. 2008;21(3):573-7. | |||||
| REF 47 | Can the anti-inflammatory potential of PDE4 inhibitors be realized: guarded optimism or wishful thinking . Br J Pharmacol. 2008 October; 155(3): 288-290. | |||||
| REF 48 | Orexo announces Phase IIa data on OX914 in rhinitis. Orexo. 24th March, 2009. | |||||
| REF 49 | Effects of piclamilast, a selective phosphodiesterase-4 inhibitor, on oxidative burst of sputum cells from mild asthmatics and stable COPD patients. Lung. 2004;182(6):369-77. | |||||
| REF 50 | WO patent application no. 2012,1109,46, Pharmaceutical composition comprising the pde4 enzyme inhibitor revamilast and a disease modifying agent, preferably methotrexate . | |||||
| REF 51 | Potential role of phosphodiesterase 7 in human T cell function: comparative effects of two phosphodiesterase inhibitors. Clin Exp Immunol. 2002 Jun;128(3):460-6. | |||||
| REF 52 | Therapy for Parkinson's Disease: What is in the Pipeline . Neurotherapeutics. 2014 January; 11(1): 24-33. | |||||
| REF 53 | GSK356278, a potent, selective, brain-penetrant phosphodiesterase 4 inhibitor that demonstrates anxiolytic and cognition-enhancing effects without inducing side effects in preclinical species. J Pharmacol Exp Ther. 2014 Jul;350(1):153-63. | |||||
| REF 54 | The effect of the novel phosphodiesterase-4 inhibitor MEM 1414 on the allergen induced responses in mild asthma. BMC Pulm Med. 2014 Oct 28;14:166. | |||||
| REF 55 | Clinical pipeline report, company report or official report of Avarx. | |||||
| REF 56 | Discovery of a substituted 8-arylquinoline series of PDE4 inhibitors: structure-activity relationship, optimization, and identification of a highly... Bioorg Med Chem Lett. 2005 Dec 1;15(23):5241-6. | |||||
| REF 57 | New substituted triaza-benzo[cd]azulen-9-ones as promising phosphodiesterase-4 inhibitors. Bioorg Med Chem Lett. 2004 Jun 21;14(12):3303-6. | |||||
| REF 58 | Inhibition of phosphodiesterase 4 reduces ethanol intake and preference in C57BL/6J mice. Front Neurosci. 2014 May 27;8:129. | |||||
| REF 59 | Pharmacokinetics and metabolism of KW-4490, a selective phosphodiesterase 4 inhibitor: difference in excretion of KW-4490 and acylglucuronide metabolites between rats and cynomolgus monkeys. Xenobiotica. 2008 May;38(5):511-26. | |||||
| REF 60 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800022894) | |||||
| REF 61 | Pharmacokinetic and pharmacodynamic profile following oral administration of the phosphodiesterase (PDE)4 inhibitor V11294A in healthy volunteers. Br J Clin Pharmacol. 2002 Nov;54(5):478-84. | |||||
| REF 62 | Can the anti-inflammatory potential of PDE4 inhibitors be realized: guarded optimism or wishful thinking. Br J Pharmacol. 2008 Oct;155(3):288-90. | |||||
| REF 63 | Synthesis and profile of SCH351591, a novel PDE4 inhibitor. Bioorg Med Chem Lett. 2002 Jun 17;12(12):1621-3. | |||||
| REF 64 | Effects of a selective PDE4 inhibitor, D-22888, on human airways and eosinophils in vitro and late phase allergic pulmonary eosinophilia in guinea pigs. Pulm Pharmacol Ther. 1998 Feb;11(1):13-21. | |||||
| REF 65 | Dual inhibition of human type 4 phosphodiesterase isostates by (R, R)-(+/-)-methyl 3-acetyl-4-[3-(cyclopentyloxy)-4-methoxyphenyl]-3- methyl-1-pyrrolidinecarboxylate. Biochemistry. 1998 May 12;37(19):6894-904. | |||||
| REF 66 | Preclinical trials in Chronic obstructive pulmonary disease in Japan (PO). 2004 | |||||
| REF 67 | 8-Substituted analogues of 3-(3-cyclopentyloxy-4-methoxy-benzyl)-8-isopropyl-adenine: highly potent and selective PDE4 inhibitors. J Med Chem. 2005 Feb 24;48(4):1237-43. | |||||
| REF 68 | Effects of alkyl substitutions of xanthine skeleton on bronchodilation. J Med Chem. 1992 Oct 30;35(22):4039-44. | |||||
| REF 69 | A new generation of phosphodiesterase inhibitors: multiple molecular forms of phosphodiesterase and the potential for drug selectivity. J Med Chem. 1985 May;28(5):537-45. | |||||
| REF 70 | Effect of orally administered KF66490, a phosphodiesterase 4 inhibitor, on dermatitis in mouse models. Int Immunopharmacol. 2009 Jan;9(1):55-62. | |||||
| REF 71 | The discovery and synthesis of highly potent subtype selective phosphodiesterase 4D inhibitors. Bioorg Med Chem Lett. 2010 Sep 15;20(18):5502-5. | |||||
| REF 72 | Enantiomer discrimination illustrated by the high resolution crystal structures of type 4 phosphodiesterase. J Med Chem. 2006 Mar 23;49(6):1867-73. | |||||
| REF 73 | In silico search for multi-target anti-inflammatories in Chinese herbs and formulas. Bioorg Med Chem. 2010 Mar 15;18(6):2204-2218. | |||||
| REF 74 | Comparison of recombinant human PDE4 isoforms: interaction with substrate and inhibitors. Cell Signal. 1998 Jun;10(6):427-40. | |||||
| REF 75 | First dual M3 antagonists-PDE4 inhibitors: synthesis and SAR of 4,6-diaminopyrimidine derivatives. Bioorg Med Chem Lett. 2006 Apr 1;16(7):1834-9. | |||||
| REF 76 | Design, synthesis, and biological evaluation of tetrahydroisoquinolines derivatives as novel, selective PDE4 inhibitors for antipsoriasis treatment. Eur J Med Chem. 2021 Feb 5;211:113004. | |||||
| REF 77 | Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004 Dec;12(12):2233-47. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

