Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T00442
|
|||||
| Target Name |
HUMAN catechol-O-methyl-transferase (COMT)
|
|||||
| Synonyms |
S-COMT; MB-COMT; Catechol-O-methyltransferase; COMT
Click to Show/Hide
|
|||||
| Gene Name |
COMT
|
|||||
| Function |
Human protein catechol-O-methyltransferase interacts with SARS-CoV-2 Nsp7 protein with high significance, which indicates COMT as a potential therapeutic target.
Click to Show/Hide
|
|||||
| BioChemical Class |
Methyltransferase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 2.1.1.6
|
|||||
| Sequence |
MPEAPPLLLAAVLLGLVLLVVLLLLLRHWGWGLCLIGWNEFILQPIHNLLMGDTKEQRIL
NHVLQHAEPGNAQSVLEAIDTYCEQKEWAMNVGDKKGKIVDAVIQEHQPSVLLELGAYCG YSAVRMARLLSPGARLITIEINPDCAAITQRMVDFAGVKDKVTLVVGASQDIIPQLKKKY DVDTLDMVFLDHWKDRYLPDTLLLEECGLLRKGTVLLADNVICPGAPDFLAHVRGSSCFE CTHYQSFLEYREVVDGLEKAIYKGPGSEAGP Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Ademetionine | Ligand Info | |||||
| Structure Description | Crystal Structure of Human 108M Catechol O-methyltransferase bound with S-adenosylmethionine and inhibitor dinitrocatechol | PDB:3BWY | ||||
| Method | X-ray diffraction | Resolution | 1.30 Å | Mutation | No | [2] |
| PDB Sequence |
GDTKEQRILN
11 HVLQHAEPGN21 AQSVLEAIDT31 YCEQKEWAMN41 VGDKKGKIVD51 AVIQEHQPSV 61 LLELGAYCGY71 SAVRMARLLS81 PGARLITIEI91 NPDCAAITQR101 MVDFAGMKDK 111 VTLVVGASQD121 IIPQLKKKYD131 VDTLDMVFLD141 HWKDRYLPDT151 LLLEECGLLR 161 KGTVLLADNV171 ICPGAPDFLA181 HVRGSSCFEC191 THYQSFLEYR201 EVVDGLEKAI 211 YKGP
|
|||||
|
|
MET40
3.440
ASN41
3.721
VAL42
2.838
GLU64
3.935
GLY66
2.904
ALA67
3.582
TYR68
3.324
GLY70
4.543
TYR71
3.248
SER72
2.751
ILE89
3.692
GLU90
2.670
ILE91
3.221
|
|||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Ligand Name: Sinefungin | Ligand Info | |||||
| Structure Description | Crystal structure of Human soluble catechol O-methyltransferase in complex with 3,5-dinitrocatechol and Sinefungin | PDB:6I3D | ||||
| Method | X-ray diffraction | Resolution | 1.45 Å | Mutation | No | [3] |
| PDB Sequence |
GDTKEQRILN
11 HVLQHAEPGN21 AQSVLEAIDT31 YCEQKEWAMN41 VGDKKGKIVD51 AVIQEHQPSV 61 LLELGAYCGY71 SAVRMARLLS81 PGARLITIEI91 NPDCAAITQR101 MVDFAGVKDK 111 VTLVVGASQD121 IIPQLKKKYD131 VDTLDMVFLD141 HWKDRYLPDT151 LLLEECGLLR 161 KGTVLLADNV171 ICPGAPDFLA181 HVRGSSCFEC191 THYQSFLEYR201 EVVDGLEKAI 211 YKGP
|
|||||
|
|
MET40
3.367
ASN41
3.669
VAL42
2.841
GLU64
3.962
LEU65
4.995
GLY66
2.875
ALA67
3.530
TYR68
3.208
GLY70
4.489
TYR71
3.302
SER72
2.746
ILE89
3.591
GLU90
2.655
ILE91
3.247
|
|||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Steroid hormone biosynthesis | hsa00140 | Affiliated Target |

|
| Class: Metabolism => Lipid metabolism | Pathway Hierarchy | ||
| Tyrosine metabolism | hsa00350 | Affiliated Target |

|
| Class: Metabolism => Amino acid metabolism | Pathway Hierarchy | ||
| Dopaminergic synapse | hsa04728 | Affiliated Target |
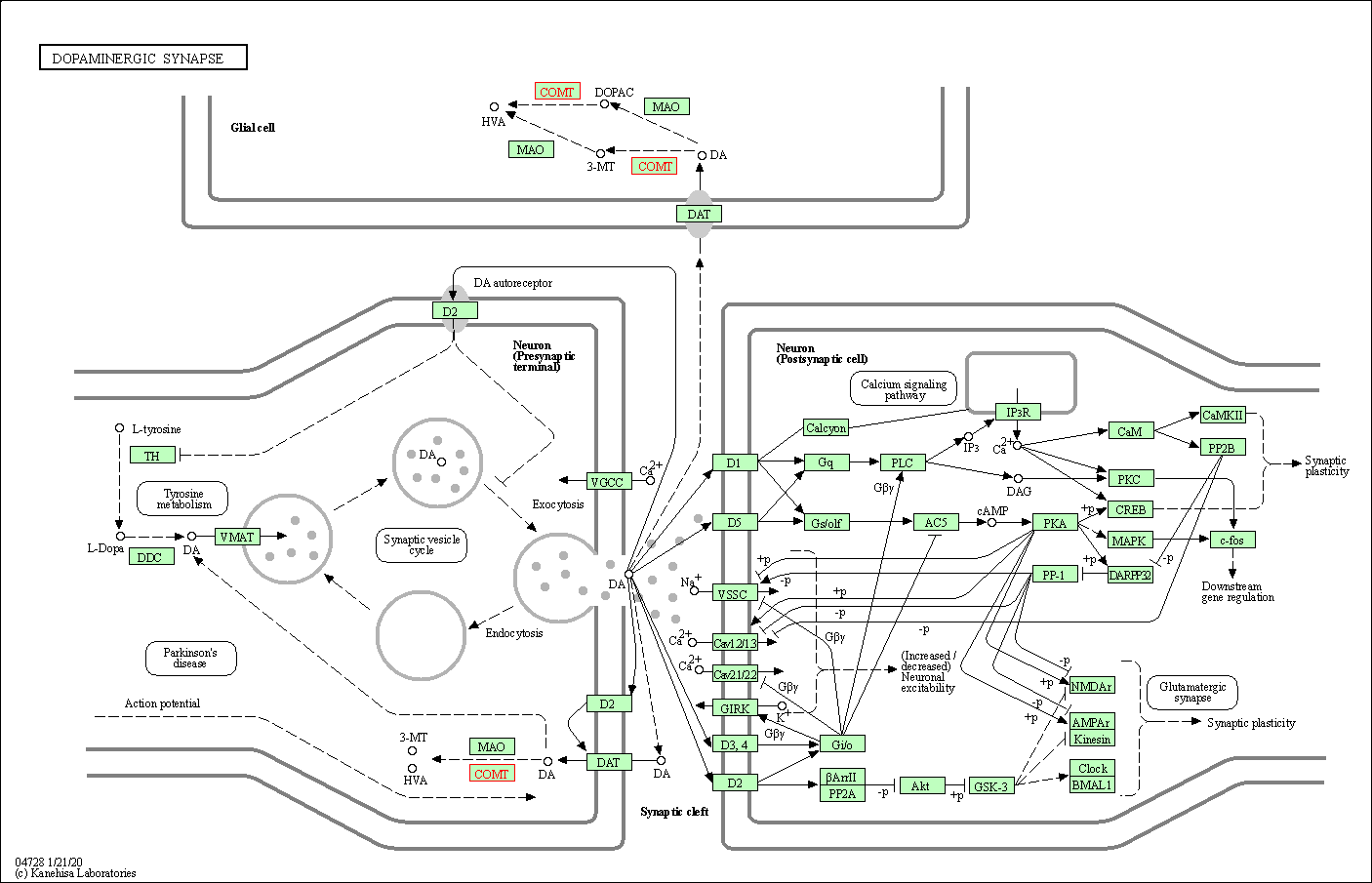
|
| Class: Organismal Systems => Nervous system | Pathway Hierarchy | ||
| Degree | 7 | Degree centrality | 7.52E-04 | Betweenness centrality | 5.35E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 1.75E-01 | Radiality | 1.28E+01 | Clustering coefficient | 2.38E-01 |
| Neighborhood connectivity | 5.71E+00 | Topological coefficient | 2.09E-01 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | A SARS-CoV-2 Protein Interaction Map Reveals Targets for Drug Repurposing. Nature. 2020 Apr 30. doi: 10.1038/s41586-020-2286-9. | |||||
| REF 2 | Crystal structures of human 108V and 108M catechol O-methyltransferase. J Mol Biol. 2008 Jun 27;380(1):120-30. | |||||
| REF 3 | Equatorial Active Site Compaction and Electrostatic Reorganization in Catechol-O-methyltransferase. ACS Catal. 2019 May 3;9(5):4394-4401. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

