Target expression details
| Target's General Information | |||||
|---|---|---|---|---|---|
| Target ID | T86364 | ||||
| Target Name | Vasoactive intestinal polypeptide receptor 1 (VIPR1) | ||||
| Synonyms | Vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide receptor-1; Vasoactive intestinal peptide receptor 1; VPAC1; VPAC-1; VIPR1; VIP-R-1; Pituitary adenylate cyclase activating polypeptide type II receptor; PACAP-R-2; PACAP type II receptor | ||||
| Target Type | Clinical trial | ||||
| Gene Name | VIPR1 | ||||
| Biochemical Class | GPCR secretin | ||||
| UniProt ID | VIPR1_HUMAN | ||||
| Target's Expression Profile in Disease Related Tissue between Patients and Normal People | |||||
| Disease | Chronic obstructive pulmonary disease | ||||
| Example drug | Vasoactive intestinal peptide | Phase 2 | [1], [2], [3] | ||
| Tissue | Lung tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual |
Fold-change: -0.16 Z-score: -0.14 P-value: 6.47E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
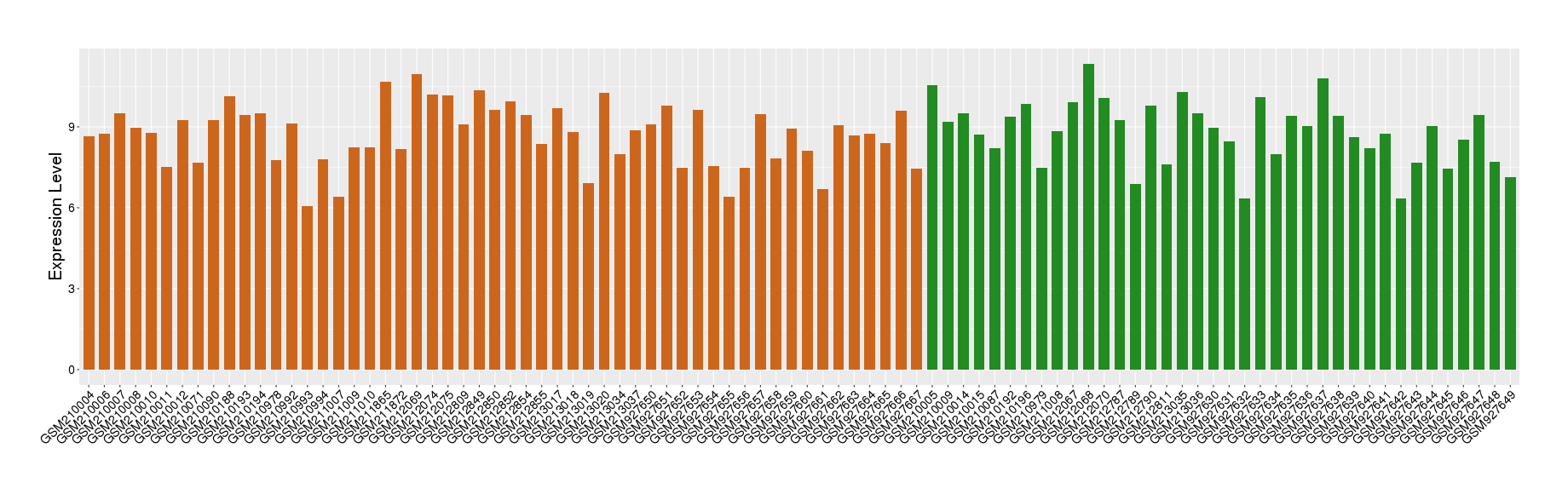
|
|||||
| Disease | Chronic obstructive pulmonary disease | ||||
| Example drug | Vasoactive intestinal peptide | Phase 2 | [1], [2], [3] | ||
| Tissue | Small airway epithelium | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual |
Fold-change: -0.12 Z-score: -0.26 P-value: 4.24E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
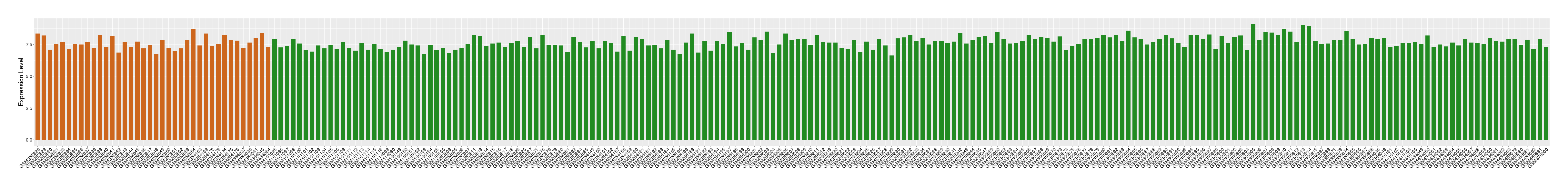
|
|||||
| Target's Expression Profile across Various Tissues of Healthy Individual | |||||
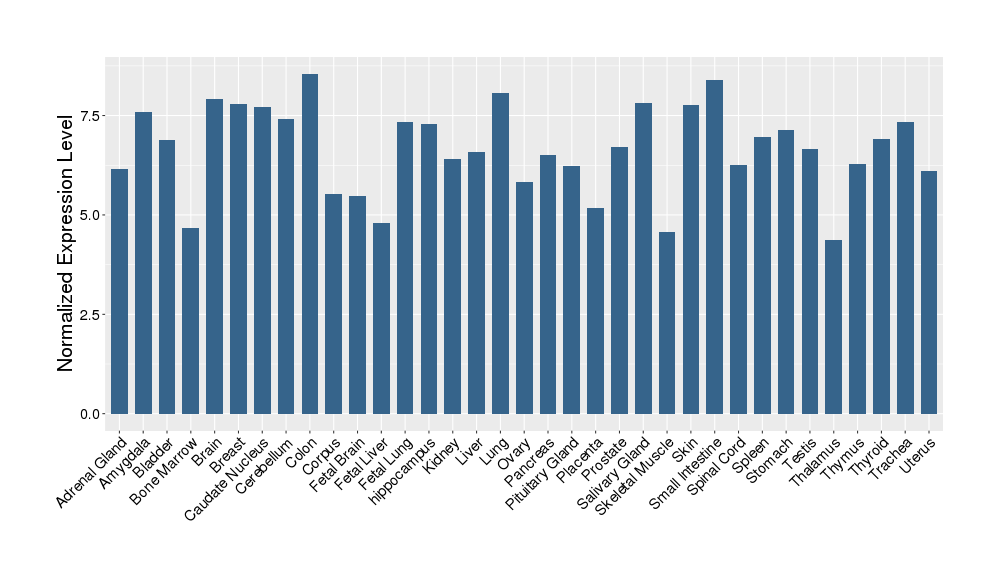
|
|||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT00464932) Vasoactive Intestinal Peptide in COPD. U.S. National Institutes of Health. | ||||
| REF 2 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | ||||
| REF 3 | NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 2013 Jan;41(Database issue):D991-5. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

