Target expression details
| Target's General Information | |||||
|---|---|---|---|---|---|
| Target ID | T22095 | ||||
| Target Name | Interleukin-17 (IL17) | ||||
| Synonyms | Interleukin-17A; IL-17A; IL-17; Cytotoxic T-lymphocyte-associated antigen 8; Cytotoxic T lymphocyte-associated antigen 8; CTLA8; CTLA-8 | ||||
| Target Type | Successful | ||||
| Gene Name | IL17A | ||||
| Biochemical Class | Cytokine: interleukin | ||||
| UniProt ID | IL17B_HUMAN||IL17C_HUMAN||IL17D_HUMAN||IL17F_HUMAN||IL17_HUMAN||IL25_HUMAN | ||||
| Target's Expression Profile in Disease Related Tissue between Patients and Normal People | |||||
| Disease | Rheumatoid arthritis | ||||
| Example drug | ABT-122 | Phase 2 | [1], [2], [3], [4] | ||
| Tissue | Synovial tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual |
Fold-change: -0.16 Z-score: -0.69 P-value: 2.91E-02 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
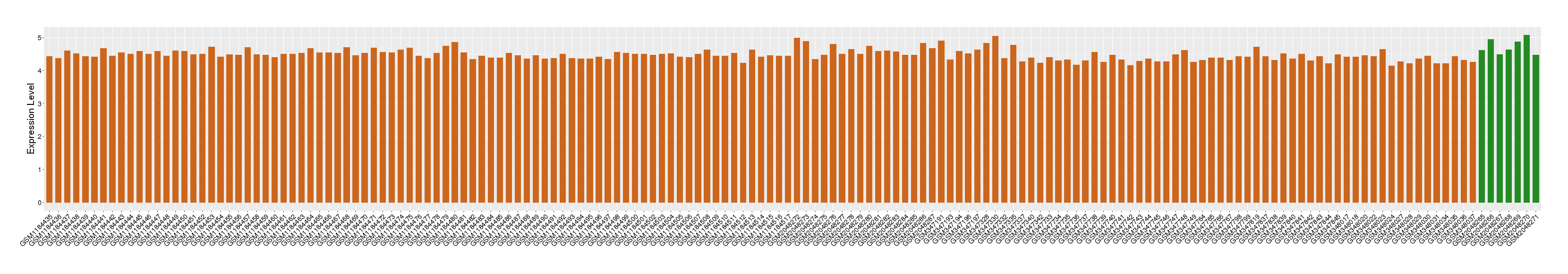
|
|||||
| Target's Expression Profile across Various Tissues of Healthy Individual | |||||
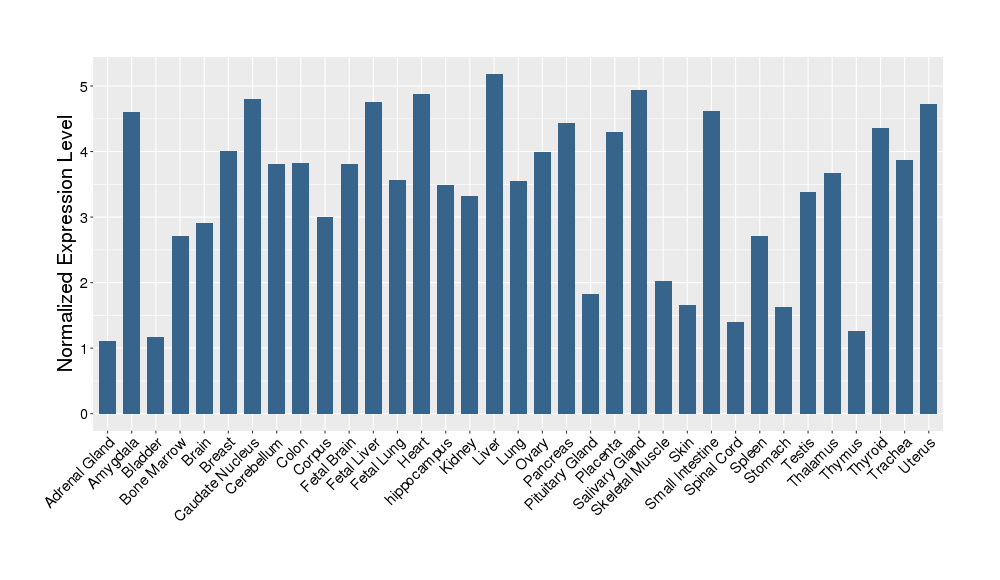
|
|||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT02349451) A Phase 2 Study to Investigate the Safety, Tolerability and Efficacy of ABT-122 in Subjects With Active Psoriatic Arthritis Who Have an Inadequate Response to Methotrexate. U.S. National Institutes of Health. | ||||
| REF 2 | ClinicalTrials.gov (NCT02433340) Phase 2, Multicenter, Open-Label Extension (OLE) Study With ABT-122 in Rheumatoid Arthritis Subjects Who Have Completed the Preceding M12-963 Study | ||||
| REF 3 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | ||||
| REF 4 | NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 2013 Jan;41(Database issue):D991-5. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

