Target expression details
| Target's General Information | |||||
|---|---|---|---|---|---|
| Target ID | T18779 | ||||
| Target Name | Mucin-1 (MUC1) | ||||
| Synonyms | Tumour-associated antigen mucin 1; Tumor-associated mucin; Tumor-associated epithelial membraneantigen; Tumor-associated epithelial membrane antigen; Polymorphic epithelial mucin; Peanut-reactive urinary mucin; PUM; PEMT; PEM; MUC-1; Krebs von den Lungen-6; KL-6; H23AG; Episialin; EMA; Carcinoma-associated mucin; Cancer antigen 15-3; CD227 antigen; CD227; CA 15-3; Breast carcinoma-associated antigen DF3 | ||||
| Target Type | Clinical trial | ||||
| Gene Name | MUC1 | ||||
| UniProt ID | MUC1_HUMAN | ||||
| Target's Expression Profile in Disease Related Tissue between Patients and Normal People | |||||
| Disease | Bipolar disorder | ||||
| Example drug | TG-4010 | Phase 2/3 | [1], [2], [3] | ||
| Tissue | Pre-frontal cortex | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual |
Fold-change: -0.07 Z-score: -0.41 P-value: 4.59E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
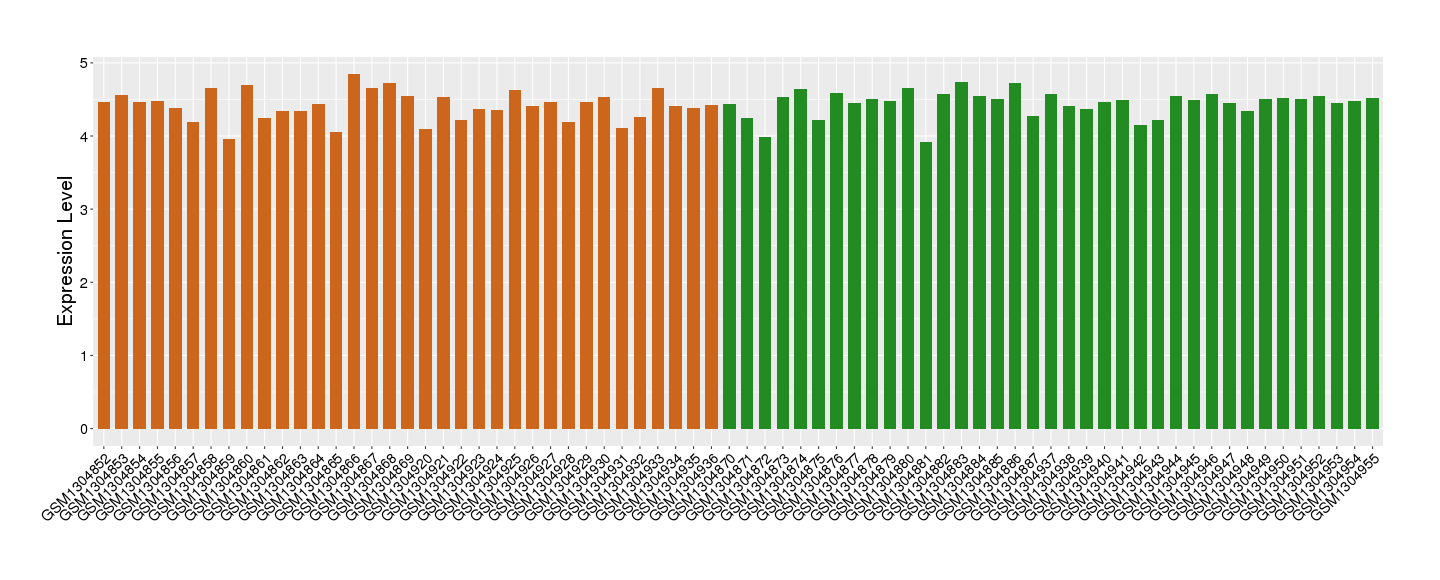
|
|||||
| Disease | Breast cancer | ||||
| Example drug | AS-1402 | Phase 2 | [2], [3], [4] | ||
| Tissue | Breast tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual |
Fold-change: 1.61 Z-score: 1.37 P-value: 1.85E-53 |
||||
| Level of differential expression between the patients in the disease section of the tissue section of the tissue adjacent to the disease section |
Fold-change: 1.9 Z-score: 1.19 P-value: 9.93E-08 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles of the patients in the normal section of the tissue adjacent to the disease section
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Disease | Lung cancer | ||||
| Example drug | Emepepimut-S | Phase 2 | [2], [3], [5] | ||
| Tissue | Lung tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual |
Fold-change: -0.72 Z-score: -1.38 P-value: 6.28E-51 |
||||
| Level of differential expression between the patients in the disease section of the tissue section of the tissue adjacent to the disease section |
Fold-change: -0.39 Z-score: -0.41 P-value: 1.41E-10 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles of the patients in the normal section of the tissue adjacent to the disease section
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Disease | Rectal cancer | ||||
| Example drug | MUC1-Poly-ICLC | Phase 2 | [2], [3], [6] | ||
| Tissue | Rectal colon tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual |
Fold-change: -0.43 Z-score: -0.83 P-value: 1.61E-01 |
||||
| Level of differential expression between the patients in the disease section of the tissue section of the tissue adjacent to the disease section |
Fold-change: -0.11 Z-score: -0.27 P-value: 1.77E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles of the patients in the normal section of the tissue adjacent to the disease section
Target gene expression profiles in the tissue of healthy individual
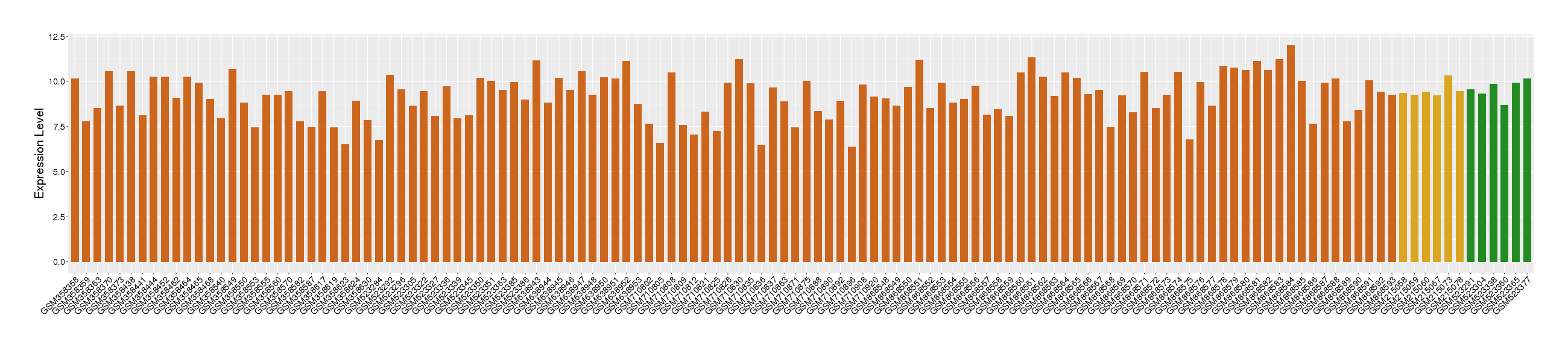
|
|||||
| Disease | Myeloma | ||||
| Example drug | ImMucin | Phase 1/2 | [2], [3], [7] | ||
| Tissue | Bone marrow | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual |
Fold-change: -0.33 Z-score: -1.54 P-value: 1.53E-02 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Disease | Acute myelocytic leukemia | ||||
| Tissue | Bone marrow | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual |
Fold-change: 0.2 Z-score: 0.38 P-value: 2.90E-04 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual

|
|||||
| Target's Expression Profile across Various Tissues of Healthy Individual | |||||
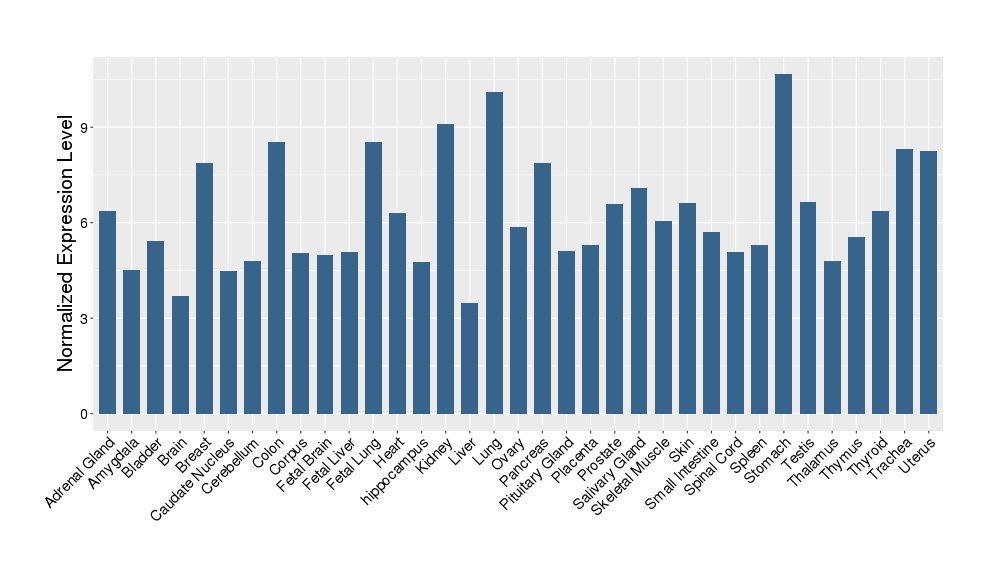
|
|||||
| References | |||||
| REF 1 | A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV Non-small cell lung cancer. J Thorac Oncol. 2008 Jul;3(7):735-44. | ||||
| REF 2 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | ||||
| REF 3 | NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 2013 Jan;41(Database issue):D991-5. | ||||
| REF 4 | Phase I dose escalation pharmacokinetic assessment of intravenous humanized anti-MUC1 antibody AS1402 in patients with advanced breast cancer. Breast Cancer Res. 2009;11(5):R73. | ||||
| REF 5 | ClinicalTrials.gov (NCT00828009) BLP25 Liposome Vaccine and Bevacizumab After Chemotherapy and Radiation Therapy in Treating Patients With Newly Diagnosed Stage IIIA or Stage IIIB Non-Small Cell LungCancer That Cannot Be Removed by Surgery. U.S. National Institutes of Health. | ||||
| REF 6 | ClinicalTrials.gov (NCT00773097) Study of the MUC1 Peptide-Poly-ICLC Adjuvant Vaccine in Individuals With Advanced Colorectal Adenoma. U.S. National Institutes of Health. | ||||
| REF 7 | ClinicalTrials.gov (NCT01232712) A Study to Assess the Safety and Efficacy of MUC1 Peptide Vaccine and hGM-CSF in Patients With MUC1-positive Tumor Malignancies. U.S. National Institutes of Health. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

