Target expression details
| Target's General Information | |||||
|---|---|---|---|---|---|
| Target ID | T14755 | ||||
| Target Name | TNF related activation protein (CD40LG) | ||||
| Synonyms | Tumor necrosis factor ligand superfamily member 5; TRAP; TNFSF5; TNF-related activation protein; T-cell antigen Gp39; T cell antigen Gp39; CD40L; CD40-L; CD40 ligand; CD154 antigen; CD154 | ||||
| Target Type | Clinical trial | ||||
| Gene Name | CD40LG | ||||
| Biochemical Class | Cytokine: tumor necrosis factor | ||||
| UniProt ID | CD40L_HUMAN | ||||
| Target's Expression Profile in Disease Related Tissue between Patients and Normal People | |||||
| Disease | Thrombocytopenia | ||||
| Example drug | IDEC-131 | Discontinued in Phase 1 | [1], [2], [3] | ||
| Tissue | Whole blood | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual |
Fold-change: 0.04 Z-score: 0.05 P-value: 3.90E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
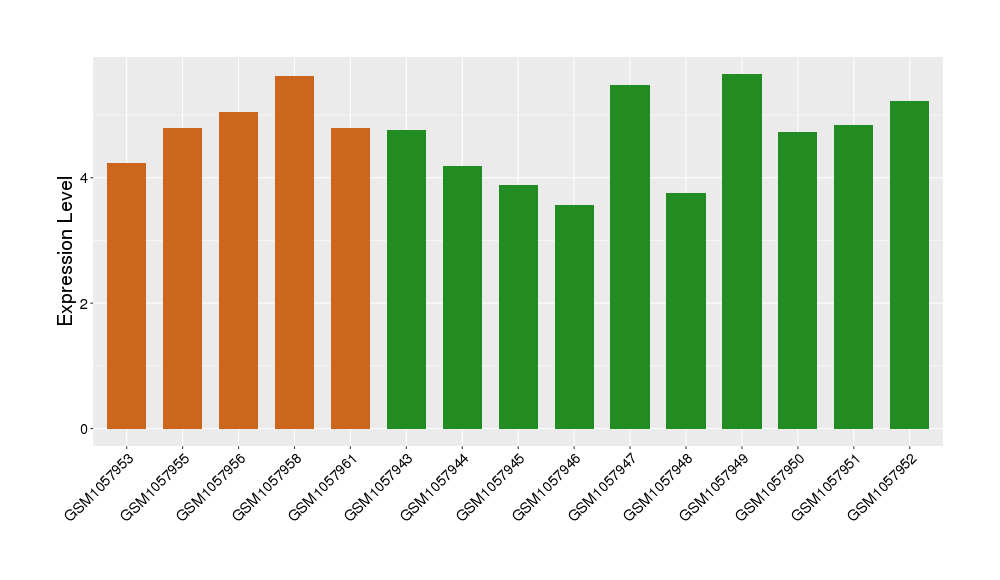
|
|||||
| Disease | Prostate cancer | ||||
| Example drug | BPX-101 | Phase 1 | [2], [3], [4] | ||
| Tissue | Prostate | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual |
Fold-change: -0.89 Z-score: -1.99 P-value: 1.81E-06 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
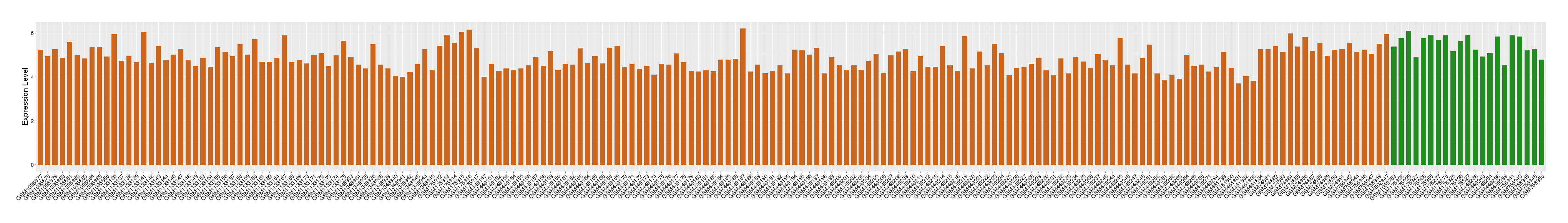
|
|||||
| Disease | Rheumatoid arthritis | ||||
| Example drug | BI 655064 | Phase 1 | [2], [3], [5] | ||
| Tissue | Synovial tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual |
Fold-change: 3.17E-03 Z-score: 7.24E-03 P-value: 8.34E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
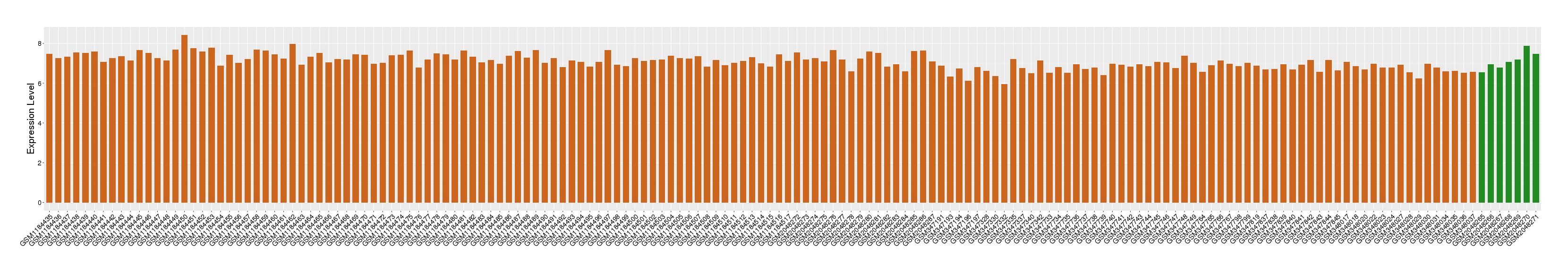
|
|||||
| Disease | Sjogren's syndrome | ||||
| Example drug | MEDI4920 | Phase 1 | [2], [3], [6] | ||
| Tissue | Salivary gland tissue | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual |
Fold-change: -0.13 Z-score: -0.7 P-value: 8.02E-01 |
||||
| Level of differential expression between the patients in the disease section of the tissue section of the tissue adjacent to the disease section |
Fold-change: -0.13 Z-score: -1.59 P-value: 3.29E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles of the patients in the normal section of the tissue adjacent to the disease section
Target gene expression profiles in the tissue of healthy individual
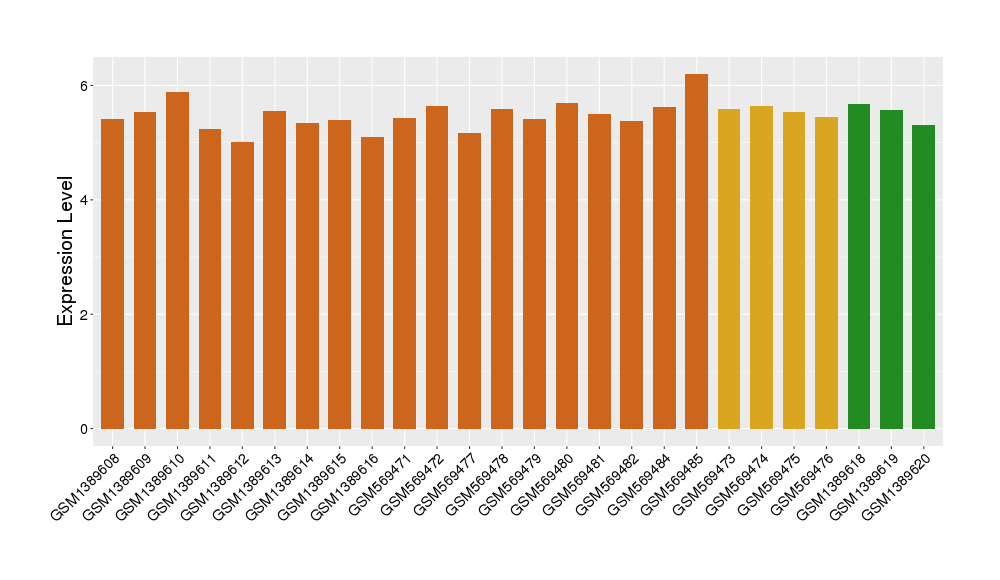
|
|||||
| Disease | Melanoma | ||||
| Tissue | Skin | ||||
| Level of differential expression between the patients in the disease section of the tissue and the tissues of healthy individual |
Fold-change: 0.32 Z-score: 0.64 P-value: 3.31E-01 |
||||
|
Target gene expression profiles of the patients in the disease section of the tissue
Target gene expression profiles in the tissue of healthy individual
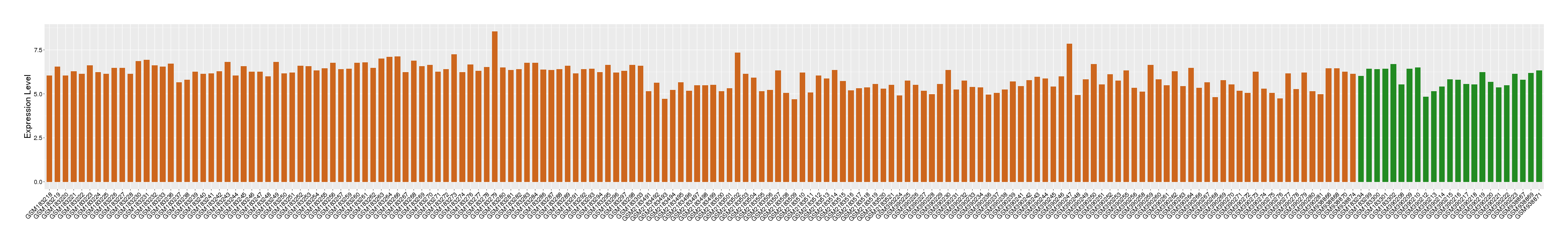
|
|||||
| Target's Expression Profile across Various Tissues of Healthy Individual | |||||
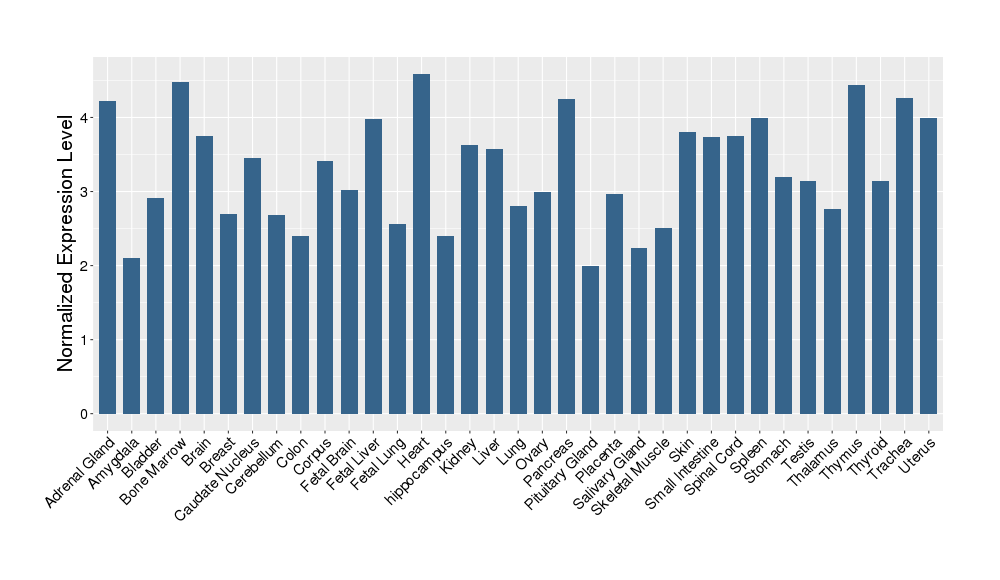
|
|||||
| References | |||||
| REF 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007783) | ||||
| REF 2 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | ||||
| REF 3 | NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 2013 Jan;41(Database issue):D991-5. | ||||
| REF 4 | ClinicalTrials.gov (NCT00868595) MTD Study of Vaccine BP-GMAX-CD1 Plus AP1903 to Treat Castrate Resistant Prostate Cancer. U.S. National Institutes of Health. | ||||
| REF 5 | ClinicalTrials.gov (NCT02009761) Multiple Rising Does Study (Subcutaneous Doses) of BI 655064 in Male and Female Patients With Chronic Primary Immune Thrombocytopenic Purpura (ITP).. U.S. National Institutes of Health. | ||||
| REF 6 | ClinicalTrials.gov (NCT02151110) A Phase 1, Randomized, Blinded, Placebo-controlled, Single-ascending Dose Study to Evaluate the Safety and Tolerability of MEDI4920 in Healthy Adults. U.S. National Institutes of Health. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

