Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DZB84T
|
|||
| Drug Name |
Maralixibat
|
|||
| Synonyms |
Lopixibat; UNII-UYB6UOF69L; Maralixibat [USAN]; 716313-53-0; UYB6UOF69L; CHEMBL363392; Maralixibat (USAN); Lopixibat cation; CHEMBL17879; 1-(4-((4-((4R,5R)-3,3-dibutyl-7-(dimethylamino)-4-hydroxy-1,1-dioxido-2,3,4,5-tetrahydrobenzo[b]thiepin-5-yl)phenoxy)methyl)benzyl)-1,4-diazabicyclo[2.2.2]octan-1-ium; LUM001 CATION; LUM-001 CATION; SCHEMBL10013954; BDBM50140282; 4-Aza-1-azoniabicyclo(2.2.2)octane, 1-((4-((4-((4R,5R)-3,3-dibutyl-7-(dimethylamino)-2,3,4,5-tetrahydro-4-hydroxy-1,1-dioxido-1-benzothiepin-5-yl)phenoxy)methyl)phenyl)methyl)-; D10951; Q27291331; (4R,5R)-5-[4-[[4-(1-aza-4-azoniabicyclo[2.2.2]octan-4-ylmethyl)phenyl]methoxy]phenyl]-3,3-dibutyl-7-(dimethylamino)-1,1-dioxo-4,5-dihydro-2H-1$l^{6}-benzothiepin-4-ol; 1-{4-[4-((4R,5R)-3,3-Dibutyl-7-dimethylamino-4-hydroxy-1,1-dioxo-2,3,4,5-tetrahydro-1H-1lambda*6*-benzo[b]thiepin-5-yl)-phenoxymethyl]-benzyl}-4-aza-1-azonia-bicyclo[2.2.2]octane; chloride
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Pruritus [ICD-11: EC90; ICD-10: L29, L29.9; ICD-9: 698] | Approved | [1] | |
| Progressive familial intrahepatic cholestasis [ICD-11: 5C58.03; ICD-10: K76.8] | Phase 3 | [2] | ||
| Alagille syndrome [ICD-11: LB20.0Y] | Phase 2 | [3] | ||
| Company |
Mirum Pharmaceuticals
|
|||
| Structure |
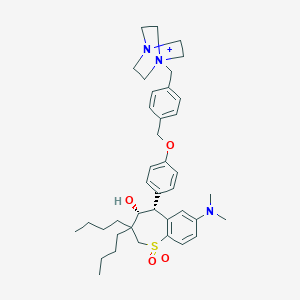 |
Download2D MOL |
||
| Formula |
C40H56N3O4S+
|
|||
| Canonical SMILES |
CCCCC1(CS(=O)(=O)C2=C(C=C(C=C2)N(C)C)C(C1O)C3=CC=C(C=C3)OCC4=CC=C(C=C4)C[N+]56CCN(CC5)CC6)CCCC
|
|||
| InChI |
1S/C40H56N3O4S/c1-5-7-19-40(20-8-6-2)30-48(45,46)37-18-15-34(41(3)4)27-36(37)38(39(40)44)33-13-16-35(17-14-33)47-29-32-11-9-31(10-12-32)28-43-24-21-42(22-25-43)23-26-43/h9-18,27,38-39,44H,5-8,19-26,28-30H2,1-4H3/q+1/t38-,39-/m1/s1
|
|||
| InChIKey |
STPKWKPURVSAJF-LJEWAXOPSA-N
|
|||
| CAS Number |
CAS 716313-53-0
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Ileal sodium/bile acid cotransporter (SLC10A2) | Target Info | Inhibitor | [4] |
| KEGG Pathway | Bile secretion | |||
| Reactome | Recycling of bile acids and salts | |||
| WikiPathways | Bile acid and bile salt metabolism | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2023. Application Number: 214662. | |||
| REF 2 | ClinicalTrials.gov (NCT04185363) An Extension Study of Maralixibat in Patients With Progressive Familial Intrahepatic Cholestasis (PFIC). U.S. National Institutes of Health. | |||
| REF 3 | ClinicalTrials.gov (NCT04729751) A Study to Evaluate the Safety and Tolerability of Maralixibat in Infant Participants With Cholestatic Liver Diseases Including Progressive Familial Intrahepatic Cholestasis (PFIC) and Alagille Syndrome (ALGS). (RISE). U.S. National Institutes of Health. | |||
| REF 4 | Ileal bile acid transporter inhibition as an anticholestatic therapeutic target in biliary atresia and other cholestatic disorders. Hepatol Int. 2020 Sep;14(5):677-689. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

