Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DXP04H
|
|||
| Drug Name |
Pirtobrutinib
|
|||
| Synonyms |
BTK inhibitor 16; 2101700-15-4; Pirtobrutinib [USAN]; UNII-JNA39I7ZVB; JNA39I7ZVB; LOXO-305; SCHEMBL19014257; WHO 11681; HY-131328; LY3527727; CS-0133286; (S)-5-Amino-3-(4-((5-fluoro-2-methoxybenzamido)methyl)phenyl)-1-(1,1,1-trifluoropropan-2-yl)-1H-pyrazole-4-carboxamide; 1H-Pyrazole-4-carboxamide, 5-amino-3-(4-(((5-fluoro-2-methoxybenzoyl)amino)methyl)phenyl)-1-((1S)-2,2,2-trifluoro-1-methylethyl)-
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Non-hodgkin lymphoma [ICD-11: 2B33.5; ICD-10: C82-C85; ICD-9: 200, 202] | Approved | [1] | |
| Chronic lymphocytic leukaemia [ICD-11: 2A82.0; ICD-10: C83.0, C91.1] | Phase 3 | [2] | ||
| Small lymphocytic lymphoma [ICD-11: 2A82.0; ICD-10: C83.0, C91.1] | Phase 3 | [2] | ||
| Company |
Eli Lilly/Innovent Biologics
|
|||
| Structure |
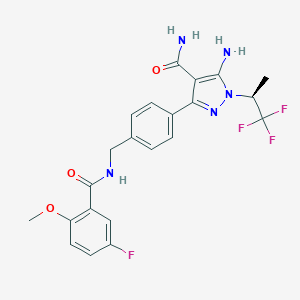 |
Download2D MOL |
||
| Formula |
C22H21F4N5O3
|
|||
| Canonical SMILES |
CC(C(F)(F)F)N1C(=C(C(=N1)C2=CC=C(C=C2)CNC(=O)C3=C(C=CC(=C3)F)OC)C(=O)N)N
|
|||
| InChI |
1S/C22H21F4N5O3/c1-11(22(24,25)26)31-19(27)17(20(28)32)18(30-31)13-5-3-12(4-6-13)10-29-21(33)15-9-14(23)7-8-16(15)34-2/h3-9,11H,10,27H2,1-2H3,(H2,28,32)(H,29,33)/t11-/m0/s1
|
|||
| InChIKey |
FWZAWAUZXYCBKZ-NSHDSACASA-N
|
|||
| CAS Number |
CAS 2101700-15-4
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2023. Application Number: 216059. | |||
| REF 2 | ClinicalTrials.gov (NCT04666038) Study of LOXO-305 Versus Investigator's Choice (IdelaR or BR) in Patients With CLL or SLL. U.S. National Institutes of Health. | |||
| REF 3 | Targeting BTK in CLL: Beyond Ibrutinib. Curr Hematol Malig Rep. 2019 Jun;14(3):197-205. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

