Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DVK21S
|
|||
| Drug Name |
BTZ-043
|
|||
| Synonyms |
BTZ043; 1161233-85-7; BTZ-043; BTZ-10526043; UNII-G55ZH52P57; 2-[(3S)-3-methyl-1,4-dioxa-8-azaspiro[4.5]decan-8-yl]-8-nitro-6-(trifluoromethyl)-1,3-benzothiazin-4-one; Bzt043; MMV676603; SCHEMBL2488829; (S)-2-(2-methyl-1,4-dioxa-8-azaspiro[4.5]decan-8-yl)-8-nitro-6-(trifluoromethyl)-4H-benzo[e][1,3]thiazin-4-one; 4H-1,3-Benzothiazin-4-one, 2-[(2S)-2-methyl-1,4-dioxa-8-azaspiro[4.5]dec-8-yl]-8-nitro-6-(trifluoromethyl)-; G55ZH52P57; 2-[(2S)-2-methyl-1,4-dioxa-8-azaspiro[4.5]decan-8-yl]-8-nitro-6-(trifluoromethyl)-4H-1,3-benzothiazin-4-one; BTZ 043; GTUIRORNXIOHQR-VIFPVBQESA-N; 4H-1,3-BENZOTHIAZIN-4-ONE, 2-((2S)-2-METHYL-1,4-DIOXA-8-AZASPIRO(4.5)DEC-8-YL)-8-NITRO-6-(TRIFLUOROMETHYL)-; BTZ043-racemate; S1097_Selleck; PBTZ 169; CHEMBL1822872; GTPL13034; DTXSID80151286; BCPP000312; EX-A1012; MFCD17215196; AKOS030526000; BCP9000457; CS-5635; AC-35368; AS-74893; BTZ 10526043; HY-13579; EN300-23853592; Q27278777; [2-[(3S)-3-methyl-1,4-dioxa-8-azaspiro[4.5]decan-8-yl]-4-oxo-6-(trifluoromethyl)-1,3-benzothiazin-8-yl]azinic acid; 2-((2S)-2-METHYL-1,4-DIOXA-8-AZASPIRO(4.5)DECAN-8-YL)-8-NITRO-6-TRIFLUOROMETHYL-4H-1,3-BENZOTHIAZIN-4-ONE
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Tuberculosis [ICD-11: 1B10-1B14] | Phase 2 | [1] | |
| Company |
TB
|
|||
| Structure |
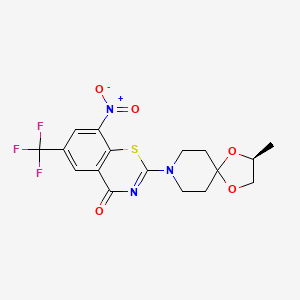 |
Download2D MOL |
||
| Formula |
C17H16F3N3O5S
|
|||
| Canonical SMILES |
CC1COC2(O1)CCN(CC2)C3=NC(=O)C4=C(S3)C(=CC(=C4)C(F)(F)F)[N+](=O)[O-]
|
|||
| InChI |
InChI=1S/C17H16F3N3O5S/c1-9-8-27-16(28-9)2-4-22(5-3-16)15-21-14(24)11-6-10(17(18,19)20)7-12(23(25)26)13(11)29-15/h6-7,9H,2-5,8H2,1H3/t9-/m0/s1
|
|||
| InChIKey |
GTUIRORNXIOHQR-VIFPVBQESA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Mycobacterium Decaprenylphosphoryl-beta-D-ribose oxidase (McyB dprE1) | Target Info | . | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04044001) A Prospective Phase Ib/IIa, Active-controlled, Randomized, Open-label Study to Evaluate the Safety, Tolerability, Extended Early Bactericidal Activity and Pharmacokinetics of Multiple Oral Doses of BTZ-043 Tablets in Subjects With Newly Diagnosed, Uncomplicated, Smear-positive, Drug-susceptible Pulmonary Tuberculosis. U.S.National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of TB | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

